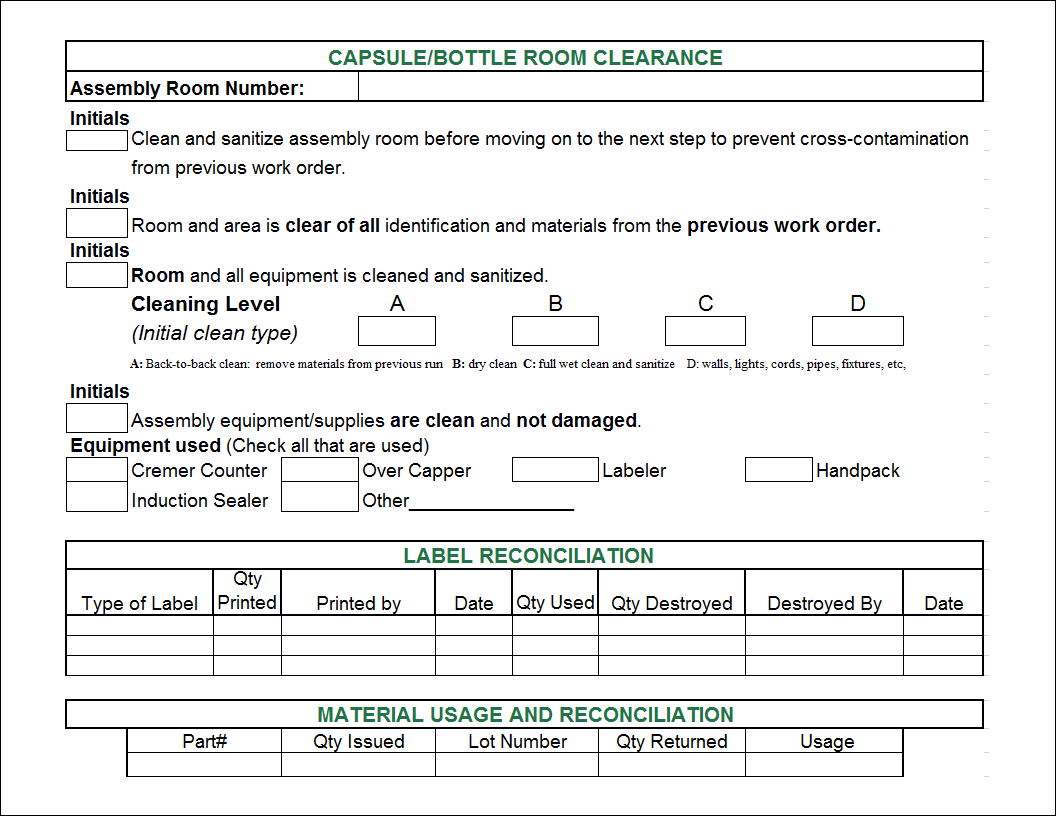
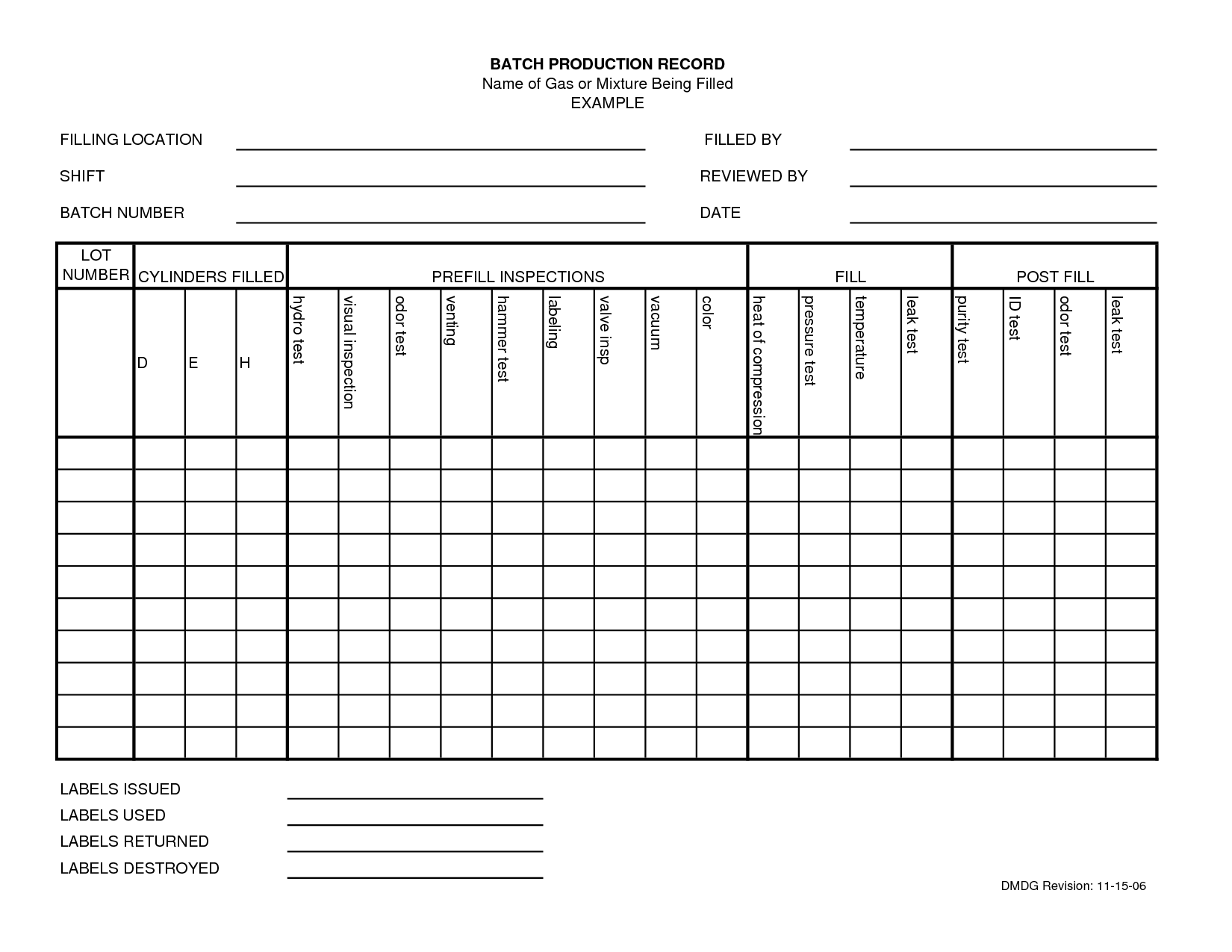
Batch Record Template
Batch Record Template - What is a batch record? Web a batch record is a comprehensive set of documents that outlines all aspects of the manufacturing process for a particular batch. Inspect the approval signatures in the batch record. Web a batch manufacturing record (bmr) is a comprehensive document that outlines the entire production process for a specific batch of a product. A batch record is the collection of data related to the manufacturing of a product batch, detailing the processing dates, the lots and quantities of the raw materials used, the staff involved, and the equipment utilized. This free batch manufacturing tool will allow you to record all the important details about your production batch for true traceability. Web in this post, we’ll review the importance of the master batch record and why more pharmaceutical manufacturers are turning to digital solutions to help improve the way they manage their compliance records. An efficient bmr can help reduce medication shortages caused by production delays or quality control issues, which can account for up to 66% of medicine shortages. It includes details about ingredients and supplies used, equipment settings,. Regulatory bodies like the fda. Web download our free batch manufacturing spreadsheet template for excel, numbers and google sheets. It serves as a detailed guideline for manufacturers to follow in order to ensure consistency and quality control throughout the manufacturing process. Learn how to create a bmr and download a free template. Obtain a copy of the batch record. Verify materials used align with the manufacturing instruction. It details the ingredients, manufacturing process, yield reconciliation, and quality checks for a specific batch. Review the dates and times of the batch production. Web download excel batch record templates designed for blending, encapsulation, tablet compression and packaging. Web following simple steps such as listing all the necessary information, choosing a suitable format, creating a master template, and maintaining detailed documentation will help you produce an accurate batch record. An efficient bmr can help reduce medication shortages caused by production delays or quality control issues, which can account for up to 66% of medicine shortages. Contain comprehensive details related to the production and quality control of that specific batch. This role is not just critical but also mandatory. Web batch manufacturing record is a written document from the batch that is prepared during the pharmaceutical manufacturing process. Web a batch record is a detailed document outlining the full production process of a specific product batch, aimed at guaranteeing its quality and safety. Ensure batch record is complete. Follow the master manufacturing record guidelines. Prepare a bpr for every batch of dietary supplements manufactured. A batch manufacturing record, or bmr, is a document containing the details of the manufacture of each product batch, across the whole manufacturing process. It contains actual data of the batch manufacturing and whole manufacturing process step by step. It serves as a detailed guideline for manufacturers to follow in order to ensure consistency and quality control throughout the manufacturing process. Learn how to create a bmr and download a free template. What is a batch record? Web a batch record is a complete history of each product, including the raw materials and equipment used, procedures followed, and quality records. Contain comprehensive details related to the production and quality control of that specific batch. This role is not just critical but. It details the ingredients, manufacturing process, yield reconciliation, and quality checks for a specific batch. Web a batch manufacturing record (bmr) is a comprehensive document that outlines the entire production process for a specific batch of a product. Obtain a copy of the batch record. Web batch manufacturing record is a written document from the batch that is prepared during. Web following simple steps such as listing all the necessary information, choosing a suitable format, creating a master template, and maintaining detailed documentation will help you produce an accurate batch record. Follow the master manufacturing record guidelines. The manufacturing process involves several steps like dispensing ingredients, pulverizing, sifting, mixing, drying, granulating, tablet punching, printing. It contains actual data of the. Inspect the approval signatures in the batch record. The manufacturing process involves several steps like dispensing ingredients, pulverizing, sifting, mixing, drying, granulating, tablet punching, printing. What is a batch record? Web 21 cfr 111.255 defines the requirements for your batch production records: Regulatory bodies like the fda. Follow the master manufacturing record guidelines. Review the dates and times of the batch production. It details the ingredients, manufacturing process, yield reconciliation, and quality checks for a specific batch. The manufacturing process involves several steps like dispensing ingredients, pulverizing, sifting, mixing, drying, granulating, tablet punching, printing. Web this template allows batch managers to record important details such as batch. Web essentially, the bmr is the template for producing a drug and so it must be followed with precision and care. Contain comprehensive details related to the production and quality control of that specific batch. Web sop for preparation, review, approval, issuance, maintenance, and archival of controlled master batch record (mbr) throughout the product lifecycle. Follow the master manufacturing record. Web 21 cfr 111.255 defines the requirements for your batch production records: Web essentially, the bmr is the template for producing a drug and so it must be followed with precision and care. It details the ingredients, manufacturing process, yield reconciliation, and quality checks for a specific batch. Learn how to create a bmr and download a free template. Regulatory. What is a batch record? Web a batch manufacturing record (bmr) is an important document for chemical and process manufacturers. It contains actual data of the batch manufacturing and whole manufacturing process step by step. Inspect the approval signatures in the batch record. Review the dates and times of the batch production. Web a batch manufacturing record (bmr) is an important document for chemical and process manufacturers. Web download our free batch manufacturing spreadsheet template for excel, numbers and google sheets. Web a batch record is a detailed document outlining the full production process of a specific product batch, aimed at guaranteeing its quality and safety. A batch manufacturing record, or bmr,. Follow the master manufacturing record guidelines. Web essentially, the bmr is the template for producing a drug and so it must be followed with precision and care. Web 21 cfr 111.255 defines the requirements for your batch production records: Web a batch manufacturing record (bmr) is a comprehensive document that outlines the entire production process for a specific batch of. An efficient bmr can help reduce medication shortages caused by production delays or quality control issues, which can account for up to 66% of medicine shortages. Web a batch manufacturing record (bmr) is an important document for chemical and process manufacturers. Web a batch record is a complete history of each product, including the raw materials and equipment used, procedures followed, and quality records. Identify the batch record to be reviewed. Web download excel batch record templates designed for blending, encapsulation, tablet compression and packaging. Web sop for preparation, review, approval, issuance, maintenance, and archival of controlled master batch record (mbr) throughout the product lifecycle. The manufacturing process involves several steps like dispensing ingredients, pulverizing, sifting, mixing, drying, granulating, tablet punching, printing. Web download our free batch manufacturing spreadsheet template for excel, numbers and google sheets. Web 21 cfr 111.255 defines the requirements for your batch production records: Web an electronic batch record (ebr) system is a digital solution for documenting batch manufacturing processes. Web this template allows batch managers to record important details such as batch id, product name, batch size, production date, batch manager, quality check results, start time, end time, and any additional notes. Web use this batch record template to document all your manufactured product batches, their ingredients, and the sops needed to create them. It includes details about ingredients and supplies used, equipment settings,. Web in this post, we’ll review the importance of the master batch record and why more pharmaceutical manufacturers are turning to digital solutions to help improve the way they manage their compliance records. Web following simple steps such as listing all the necessary information, choosing a suitable format, creating a master template, and maintaining detailed documentation will help you produce an accurate batch record. Verify materials used align with the manufacturing instruction.Formula & Batch Records Crafter's Choice
How To Prepare A Batch Manufacturing Record Template vrogue.co
How to Prepare a Batch Manufacturing Record & Free Template (2022)
2024 Batch Manufacturing Record Definition, Template & Examples
Batch Record Template Excel
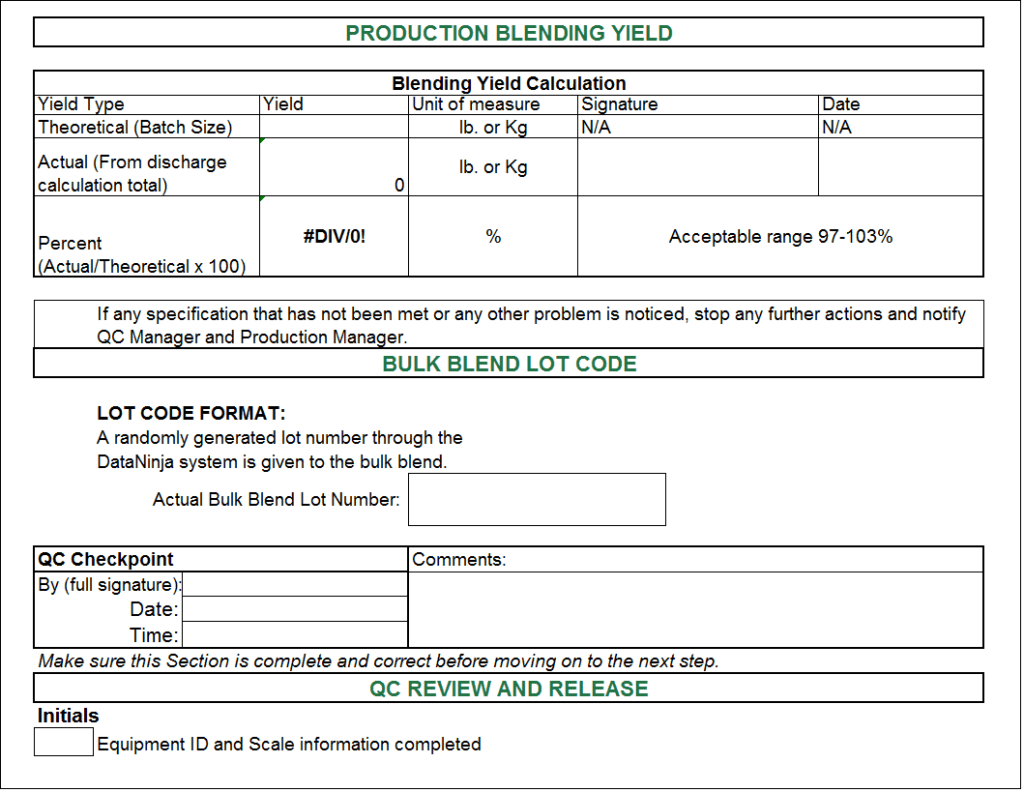
Master Batch Record Template
Batch Record Review Checklist Template/Example
Batch Sheet Template
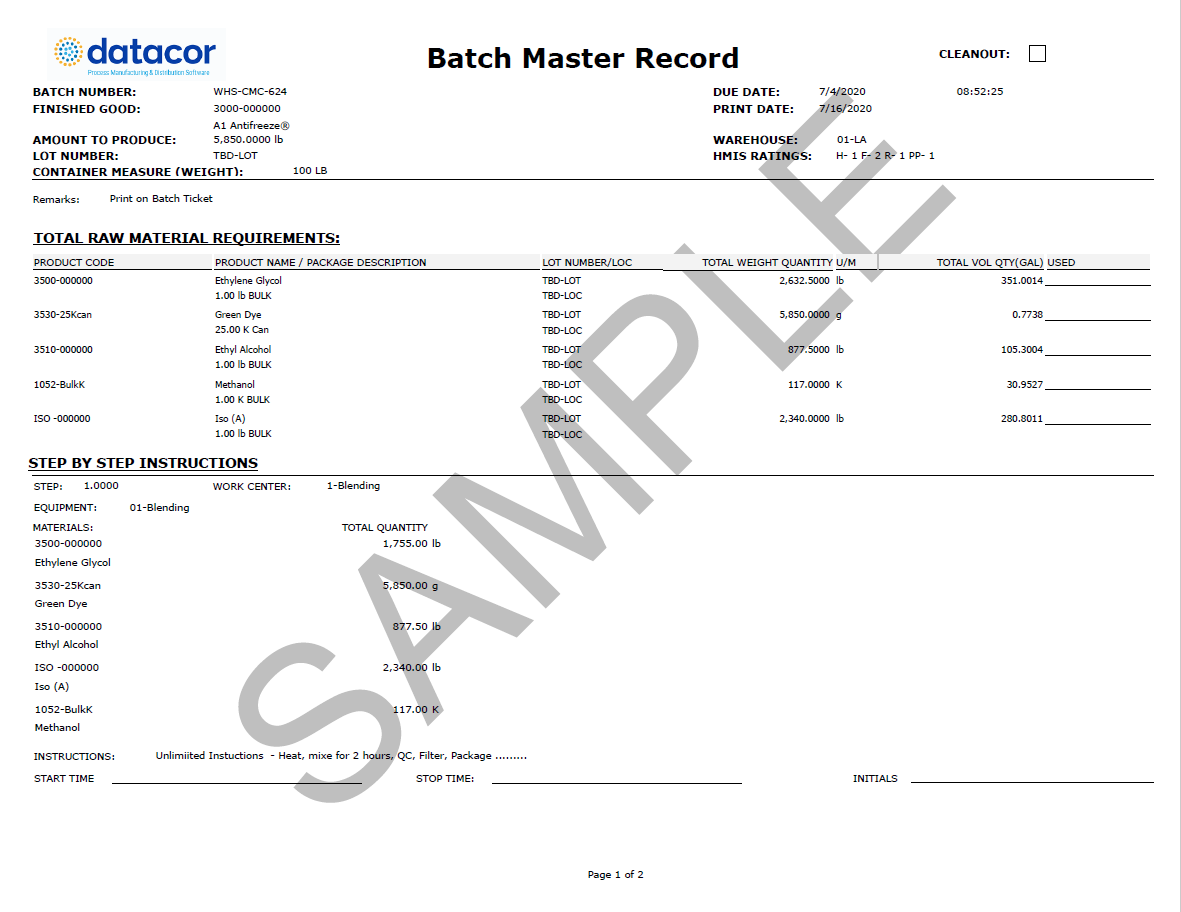
Sample of Batch Manufacturing Record (BMR) Atorvastatin PDF
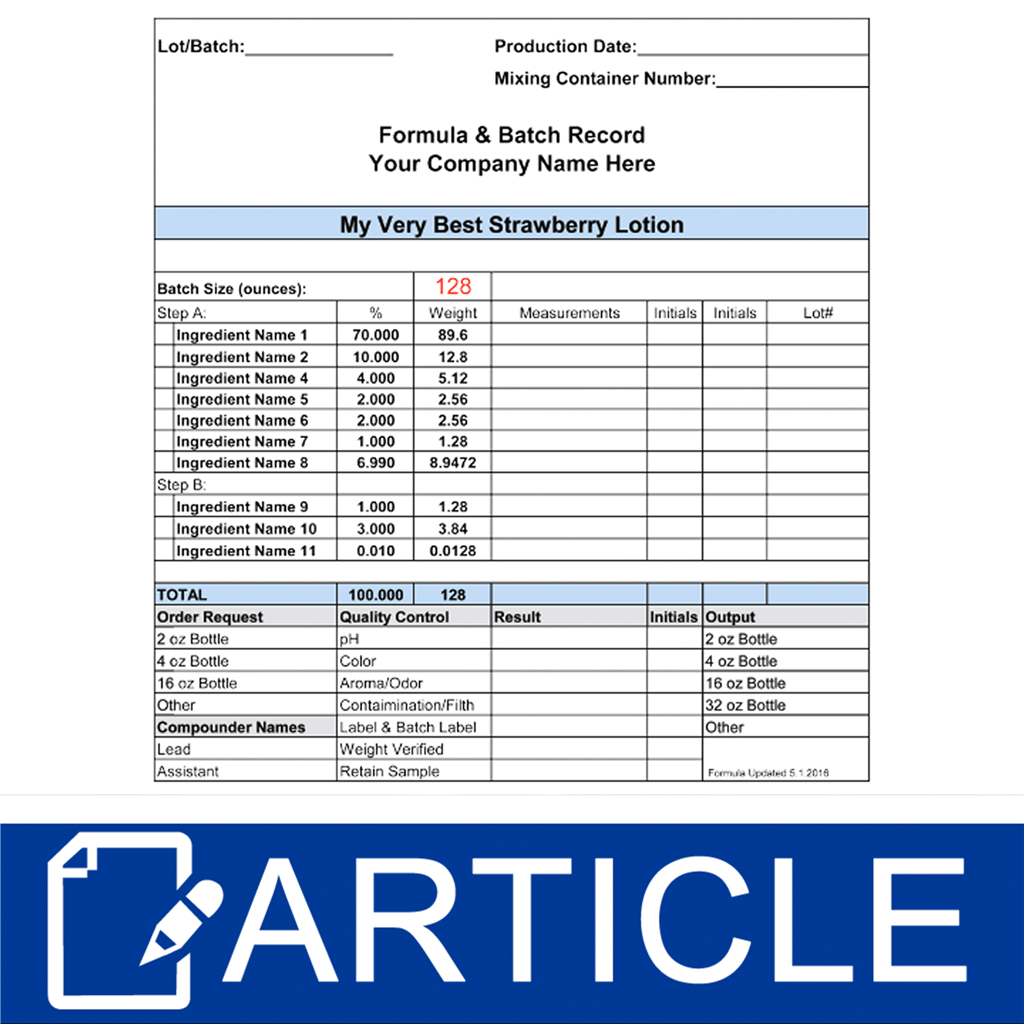
Good Manufacturing Practices Formula & Batch Records
Learn How To Create A Bmr And Download A Free Template.
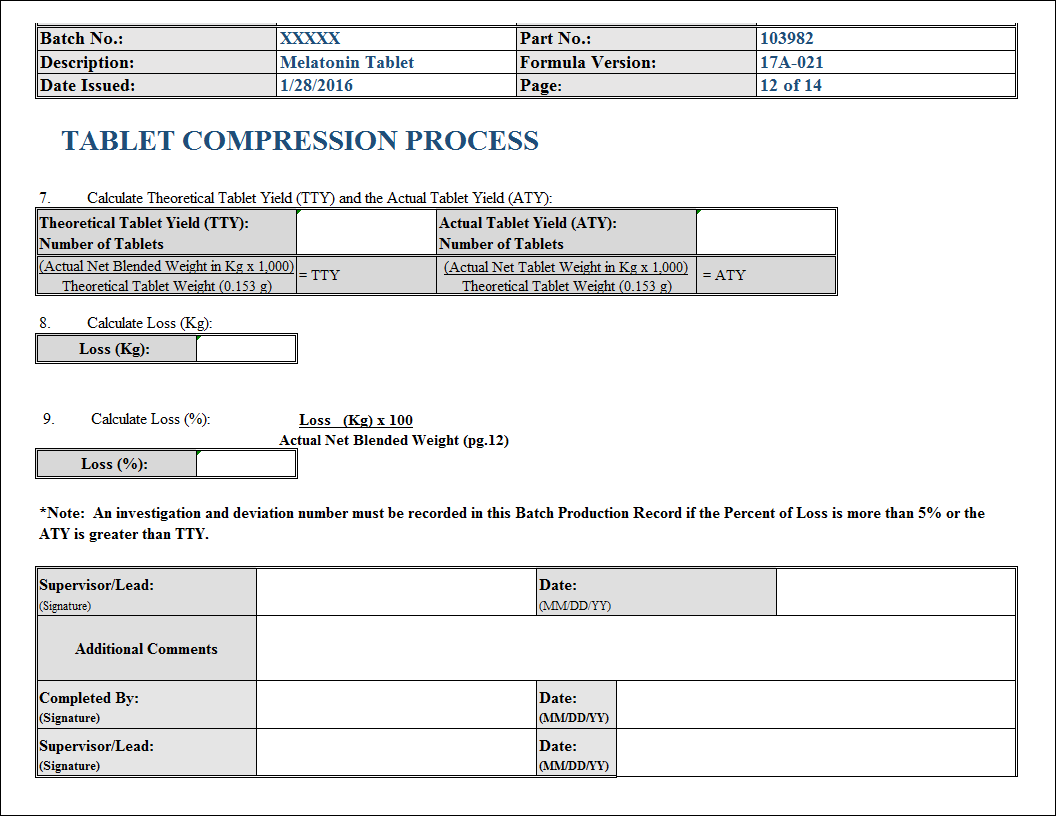
It Contains Actual Data Of The Batch Manufacturing And Whole Manufacturing Process Step By Step.
Organize And Integrate The Data.
Web Essentially, The Bmr Is The Template For Producing A Drug And So It Must Be Followed With Precision And Care.
Related Post: