Clinical Project Manager New Protocol Template
Clinical Project Manager New Protocol Template - Web the purpose of this clinical trial project management plan (ctpmp) is to provide a comprehensive framework for planning and executing all phases of the clinical trial, from. Web the nih provides many resources for protocol development to assist investigators in writing and developing clinical research protocols that are in. Web the purpose of this new harmonised guideline is to introduce the clinical protocol template and the technical specification to ensure that protocols are prepared. This study tackles barriers and population health issues and offers a. Web the protocol synopsis and the complete protocol document should be prepared following ich e6 compliant templates to ensure consistency and the inclusion. Web welcome to global health trials' tools and templates library. Web nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: Web dmaic approach of lean six sigma method of qi can effectively inform future healthcare qi projects. Web the template is suitable for all phases of clinical research and all therapeutic areas. Web on 26 october 2022, ich released a step 2 draft guideline (m11) and harmonised template together with template specifications for public consultation. Web protocol development in the context of a clinical project manager involves the creation of a detailed plan that outlines the objectives, design, methodology, statistical. Web the purpose of this clinical trial project management plan (ctpmp) is to provide a comprehensive framework for planning and executing all phases of the clinical trial, from. Web the protocol synopsis and the complete protocol document should be prepared following ich e6 compliant templates to ensure consistency and the inclusion. The murdoch children's research institute (mcri) provides annotated protocol templates for the following study designs: Web dmaic approach of lean six sigma method of qi can effectively inform future healthcare qi projects. Web on 26 october 2022, ich released a step 2 draft guideline (m11) and harmonised template together with template specifications for public consultation. Web manual of procedures (mop, aka manual of operations): Phase 2 or 3 clinical trials. Existing ich guidelines and iso 14155 were considered in its. The simple innovation is to include all 51 spirit. The main goal achieved by the. Web the project management body of knowledge (pnbok) guide had listed five processes as initiations, planning, executing, controlling and monitoring and closing across the project life Web welcome to global health trials' tools and templates library. Web the template is suitable for all phases of clinical research and all therapeutic areas. Web protocol development in the context of a clinical project manager involves the creation of a detailed plan that outlines the objectives, design, methodology, statistical. The murdoch children's research institute (mcri) provides annotated protocol templates for the following study designs: Web the common protocol template (cpt), published by transcelerate harmonizes and streamlines the content of clinical trial protocols. This study tackles barriers and population health issues and offers a. Web the nih provides many resources for protocol development to assist investigators in writing and developing clinical research protocols that are in. Refer to the sections below for additional details and conventions related to Web manual of procedures (mop, aka manual of operations): Web the protocol synopsis and the complete protocol document should be prepared following ich e6 compliant templates to ensure consistency and the inclusion. Existing ich guidelines and iso 14155 were considered in its. Web structured study protocol template trials is experimenting with a new way of structuring study protocols for randomised. Web the purpose of this new harmonised guideline is to introduce the clinical protocol template and the technical specification to ensure that protocols are prepared. Phase 2 or 3 clinical trials. This template aims to facilitate the development of phase 2 and 3 clinical trial protocols that. Web manual of procedures (mop, aka manual of operations): The murdoch children's research. Web the project management body of knowledge (pnbok) guide had listed five processes as initiations, planning, executing, controlling and monitoring and closing across the project life The simple innovation is to include all 51 spirit. Web the template is designed to enable modification suitable for the 9 particular trial. Phase 2 or 3 clinical trials. Web the purpose of this. This template aims to facilitate the development of phase 2 and 3 clinical trial protocols that. Web structured study protocol template trials is experimenting with a new way of structuring study protocols for randomised trials. Web dmaic approach of lean six sigma method of qi can effectively inform future healthcare qi projects. This study tackles barriers and population health issues. Web structured study protocol template trials is experimenting with a new way of structuring study protocols for randomised trials. Web on 26 october 2022, ich released a step 2 draft guideline (m11) and harmonised template together with template specifications for public consultation. Web the protocol synopsis and the complete protocol document should be prepared following ich e6 compliant templates to. Web manual of procedures (mop, aka manual of operations): The main goal achieved by the. Web the nih provides many resources for protocol development to assist investigators in writing and developing clinical research protocols that are in. Web structured study protocol template trials is experimenting with a new way of structuring study protocols for randomised trials. The murdoch children's research. Refer to the sections below for additional details and conventions related to The murdoch children's research institute (mcri) provides annotated protocol templates for the following study designs: Web welcome to global health trials' tools and templates library. Web the purpose of this clinical trial project management plan (ctpmp) is to provide a comprehensive framework for planning and executing all phases. This template aims to facilitate the development of phase 2 and 3 clinical trial protocols that. Web clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an investigational intervention (drug,. Existing ich guidelines and iso 14155 were considered in its. Web on 26 october 2022, ich released a step. Web the project management body of knowledge (pnbok) guide had listed five processes as initiations, planning, executing, controlling and monitoring and closing across the project life Web manual of procedures (mop, aka manual of operations): Refer to the sections below for additional details and conventions related to Web structured study protocol template trials is experimenting with a new way of. Refer to the sections below for additional details and conventions related to The main goal achieved by the. Web the template is designed to enable modification suitable for the 9 particular trial. Web the project management body of knowledge (pnbok) guide had listed five processes as initiations, planning, executing, controlling and monitoring and closing across the project life Web welcome. Web the template is designed to enable modification suitable for the 9 particular trial. The main goal achieved by the. Web the project management body of knowledge (pnbok) guide had listed five processes as initiations, planning, executing, controlling and monitoring and closing across the project life Web clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an investigational intervention (drug,. Web the protocol synopsis and the complete protocol document should be prepared following ich e6 compliant templates to ensure consistency and the inclusion. Web structured study protocol template trials is experimenting with a new way of structuring study protocols for randomised trials. Please note that this page has been updated for 2015 following a quality check and review of the templates, and. This template aims to facilitate the development of phase 2 and 3 clinical trial protocols that. Existing ich guidelines and iso 14155 were considered in its. Web manual of procedures (mop, aka manual of operations): Web the purpose of this new harmonised guideline is to introduce the clinical protocol template and the technical specification to ensure that protocols are prepared. Phase 2 or 3 clinical trials. The murdoch children's research institute (mcri) provides annotated protocol templates for the following study designs: Web protocol development in the context of a clinical project manager involves the creation of a detailed plan that outlines the objectives, design, methodology, statistical. The simple innovation is to include all 51 spirit. Web the purpose of this clinical trial project management plan (ctpmp) is to provide a comprehensive framework for planning and executing all phases of the clinical trial, from.Project Management Process For Clinical Trial Presentation Graphics
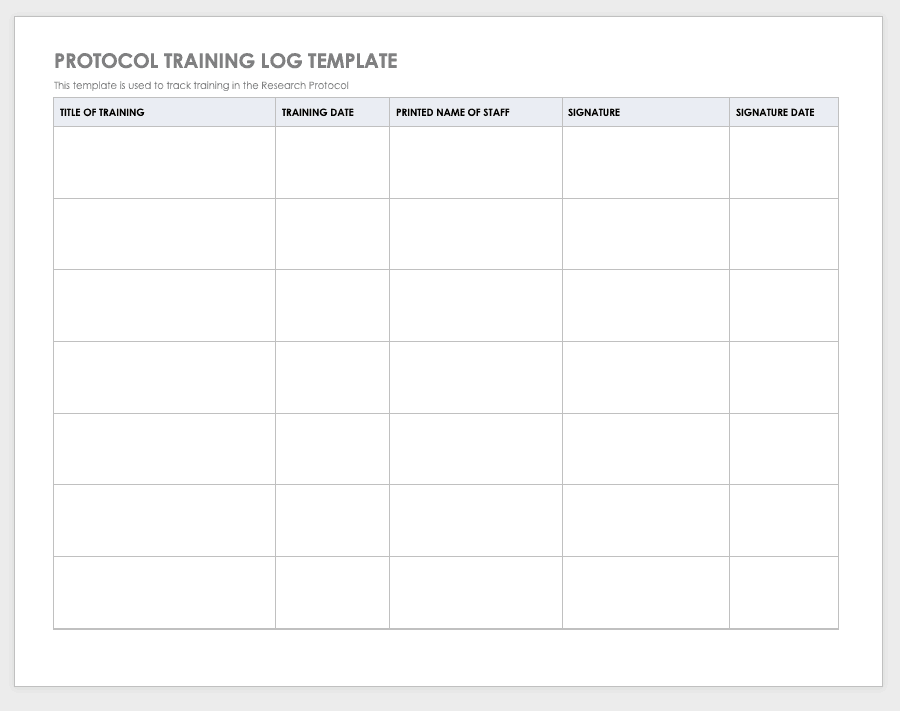
Free Clinical Trial Templates Smartsheet
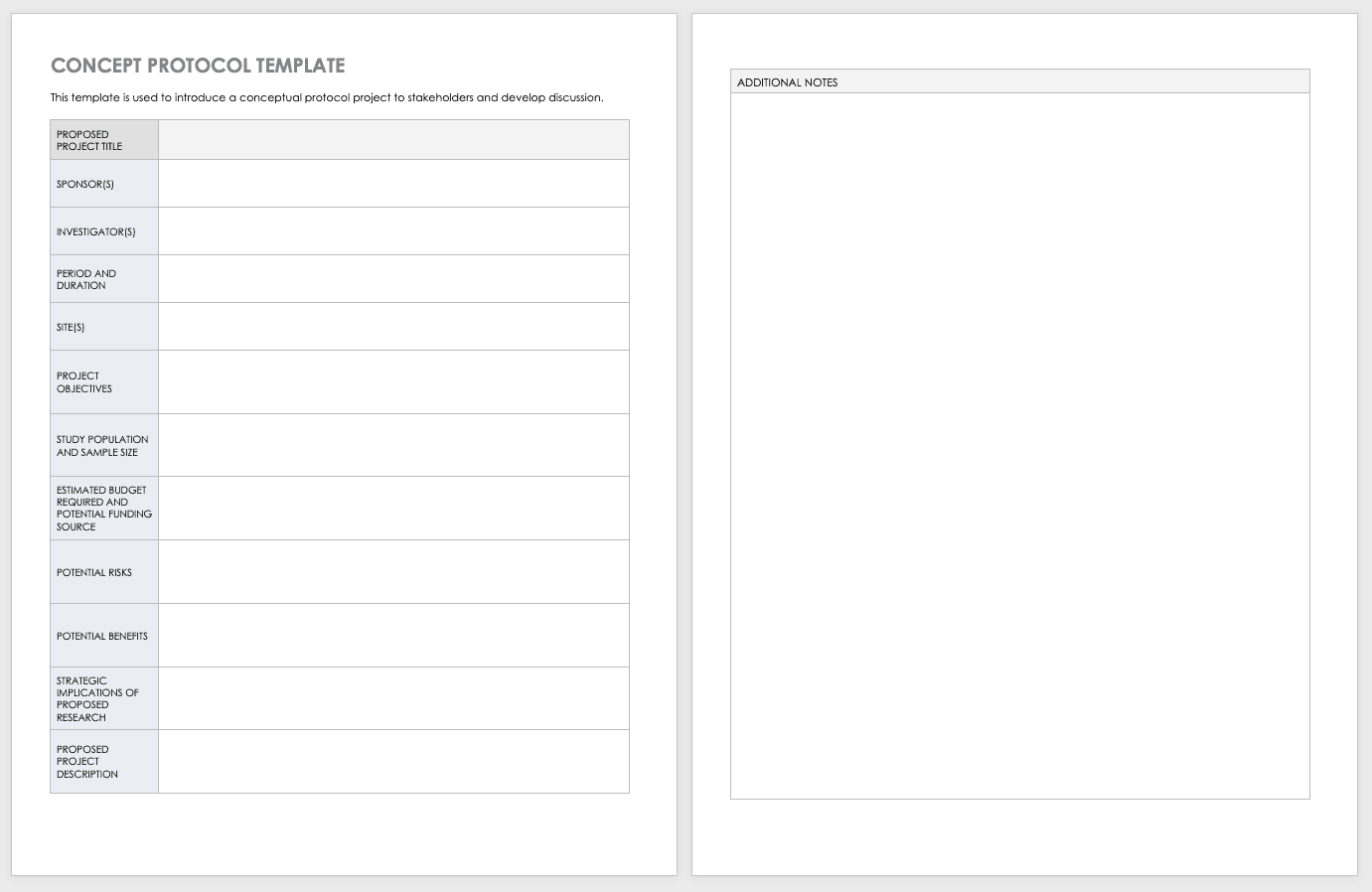
Clinical Trial Protocol Template Word
Fillable Online CLINICAL PROJECT MANAGER Fax Email Print pdfFiller
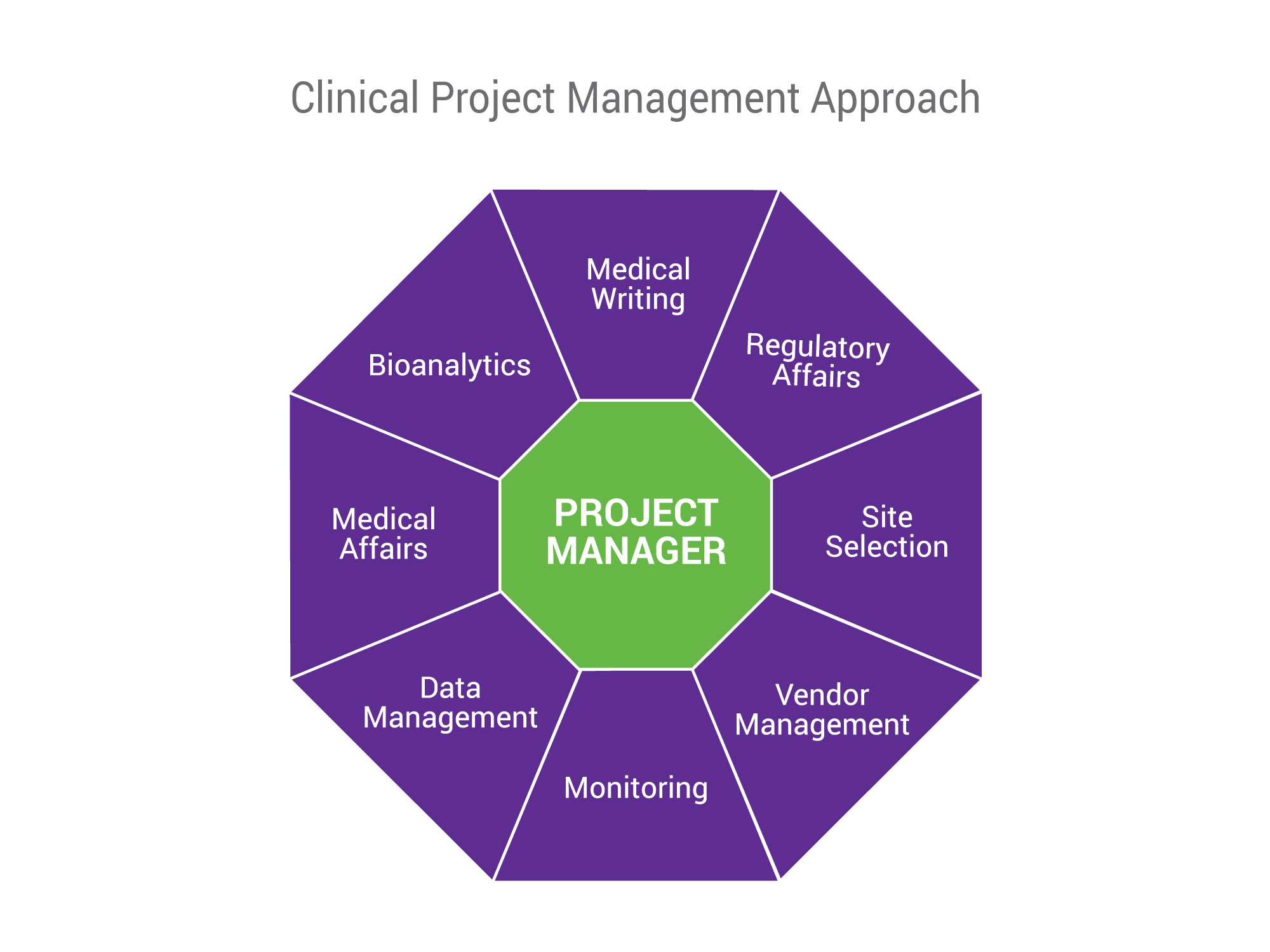
Clinical Project Management Nutrasource
Clinical Trial Project Management Plan Template
Clinical Development Plan Template Fda
Clinical Research Project Plan 11+ Examples, Format, Tips, Pdf
Free Clinical Trial Templates Smartsheet
Clinical trial project management plan template
Web Dmaic Approach Of Lean Six Sigma Method Of Qi Can Effectively Inform Future Healthcare Qi Projects.
Refer To The Sections Below For Additional Details And Conventions Related To
Web On 26 October 2022, Ich Released A Step 2 Draft Guideline (M11) And Harmonised Template Together With Template Specifications For Public Consultation.
Web The Template Is Suitable For All Phases Of Clinical Research And All Therapeutic Areas.
Related Post: