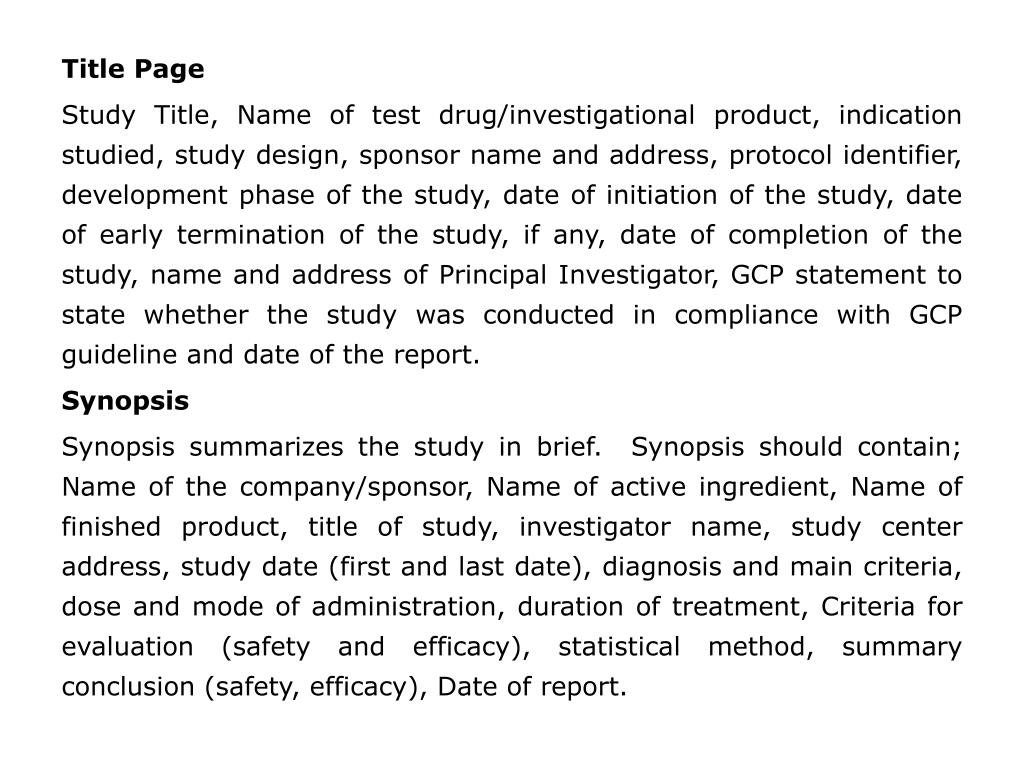
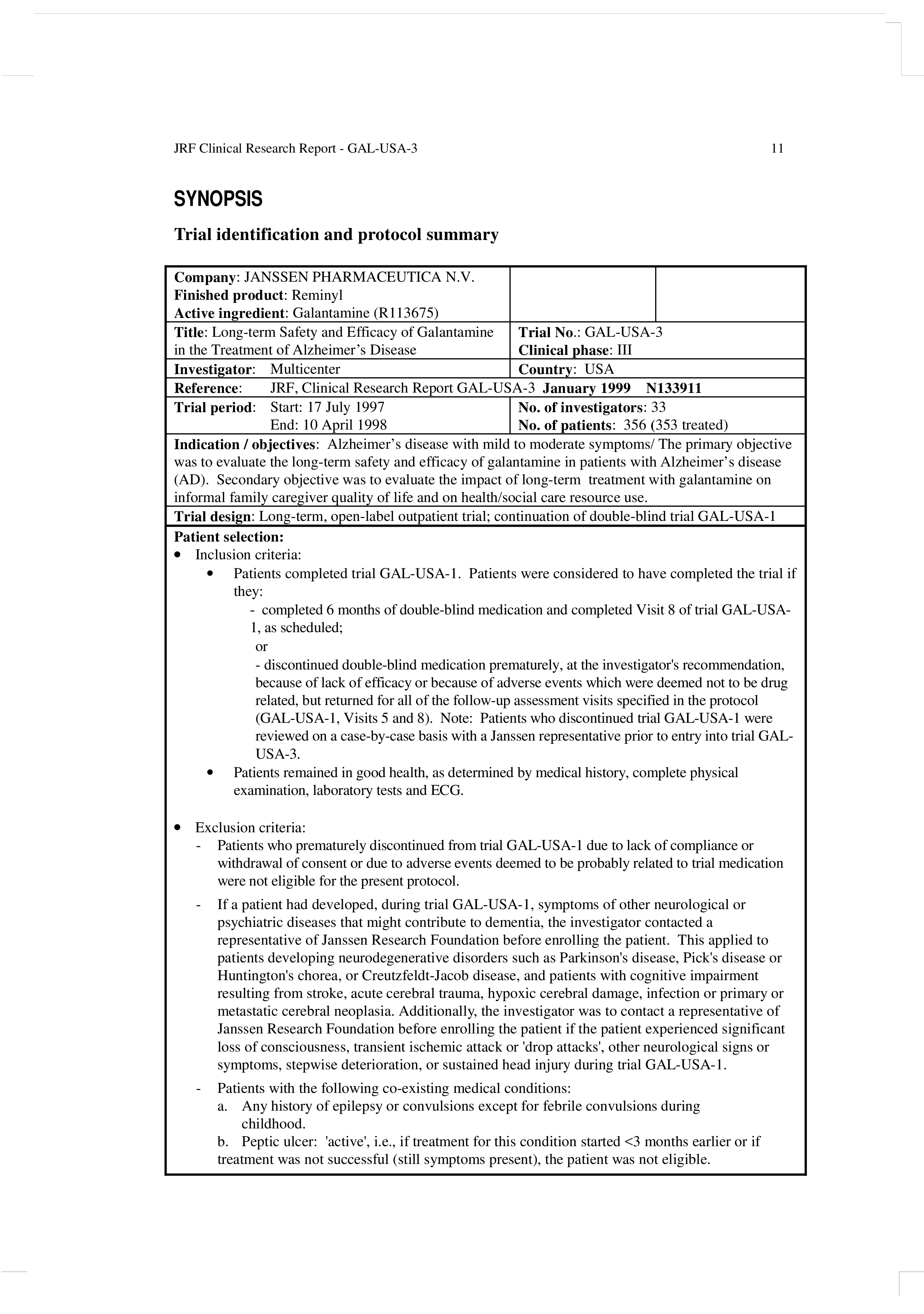
Clinical Study Report Template
Clinical Study Report Template - Web learn how to use clinical study reports (csrs) as a data source for systematic reviews. Web the clinical study report described in this guideline is an “integrated” full report of an individual study of any therapeutic, prophylactic, or diagnostic agent (referred to herein as. Learn about topics such as clinical study. The csr template includes study information, synopsis, ethics, investigators, study objectives, design, population, treatment, assessments, data analysis, and results. Web core reference is a guide for medical writers to create clinical study report (csr) content based on relevant guidelines and best practices. Web the clinical study report (csr) is arguably the most important document emerging from a clinical trial. This clinical study report summarizes a clinical trial evaluating an investigational drug. Web learn how to write effective clinical study reports (csrs) for regulatory submission in the eu, based on ich e3 and e6 guidelines. Web this document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of pharmaceuticals for human use. Web find free and adaptable templates and tools for various aspects of clinical research, such as protocol development, informed consent, data management, and ethics. Web find nih/niams tools and templates for developing clinical trial protocols, data and safety monitoring plans, and study reports. Web core reference is a guide for medical writers to create clinical study report (csr) content based on relevant guidelines and best practices. The csr template includes study information, synopsis, ethics, investigators, study objectives, design, population, treatment, assessments, data analysis, and results. Csrs summarize a study’s data and outcomes to facilitate the evaluation of a drug’s therapeutic effectiveness. Web learn how to compile a single core clinical study report acceptable to all regulatory authorities of the ich regions. Web this document provides a harmonised tripartite guideline for the structure and content of clinical study reports for registration of pharmaceuticals for human use. Web the clinical study report (csr) is arguably the most important document emerging from a clinical trial. Learn about topics such as clinical study. It covers topics such as reporting checklists, trial structure, spin, data presentation, open access and more. Web this document provides questions and answers on the ich e3 guideline, which is a guideline, not a template, for reporting clinical studies. Web this document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. Web learn how to use clinical study reports (csrs) as a data source for systematic reviews. This workshop provides an introduction to csr structure, content, benefits, challenges, and examples. Web core reference is a guide for medical writers to create clinical study report (csr) content based on relevant guidelines and best practices. Csrs summarize a study’s data and outcomes to facilitate the evaluation of a drug’s therapeutic effectiveness. Web find nih/niams tools and templates for developing clinical trial protocols, data and safety monitoring plans, and study reports. Browse by category or search by keyword to download the resources you need. Web the clinical study report (csr) is arguably the most important document emerging from a clinical trial. Web the report guide is a supplementary resource to help you write up your clinical trial report for a scientific journal. Web this document provides a core template for clinical study reports that can be used in different regions of the world. Web this document clarifies the ich e3 guidance, which is a recommendation, not a requirement, for reporting clinical study results. Web the clinical study report (csr) is arguably the most important document emerging from a clinical trial. Web learn how to write a clinical study report (csr) that meets regulatory requirements and best practices. Web core reference is a guide. It covers topics such as title page, synopsis, ethics, study objectives, design, conduct, analysis, and conclusions. Web the clinical study report (csr) is arguably the most important document emerging from a clinical trial. Web find recommendations and guidance on clinical trials and human subject protection from the international conference on harmonization (ich). Web this document provides a note for guidance. This clinical study report summarizes a clinical trial evaluating an investigational drug. Web learn how to write effective clinical study reports (csrs) for regulatory submission in the eu, based on ich e3 and e6 guidelines. Web find free templates and project plans for clinical research, including research protocol, case report form, and training log. It includes signature pages from investigators. Web this is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as an adjunctive therapy for normal tension glaucoma. Web core reference is a guide for medical writers to create clinical study report (csr) content based on relevant guidelines and best practices. Web learn how to write effective clinical study. Learn how to manage clinical trials with smartsheet software. This document covers the structure and content of clinical study reports, synopsis, ethics, study design, population, treatment, statistical plan, efficacy and safety evaluation. Web the common clinical study report (csr) template provides a common, streamlined structure to report data, adhere to global requirements, enable disclosures, and seamlessly integrate with the transcelerate. Web find free templates and project plans for clinical research, including research protocol, case report form, and training log. Web this document provides a core template for clinical study reports that can be used in different regions of the world. Learn about topics such as clinical study. This guide covers the background, planning, structure and content of csrs, with tips. Web find free templates and project plans for clinical research, including research protocol, case report form, and training log. Web core reference is a guide for medical writers to create clinical study report (csr) content based on relevant guidelines and best practices. Find useful templates, resources, and tips for the novice clinical researcher. Web the ich m11 clinical electronic structured. Web the common clinical study report (csr) template provides a common, streamlined structure to report data, adhere to global requirements, enable disclosures, and seamlessly integrate with the transcelerate cpt and sap templates. Web the clinical study report described in this guideline is an “integrated” full report of an individual study of any therapeutic, prophylactic, or diagnostic agent (referred to herein. Learn about topics such as clinical study. Web this document provides a core template for clinical study reports that can be used in different regions of the world. Browse by category or search by keyword to download the resources you need. It covers topics such as content and structure, synopsis, appendices, and terminology of clinical study reports. Web find recommendations. Web this document provides a core template for clinical study reports that can be used in different regions of the world. Web this document provides questions and answers on the ich e3 guideline, which is a guideline, not a template, for reporting clinical studies. Web the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with. Web find free templates and project plans for clinical research, including research protocol, case report form, and training log. Web download a free template for clinical study reports following ich e3 guidelines and fda recommendations. It covers topics such as ethics, study objectives, design, conduct, analysis, and reporting of clinical trials. This guide covers the background, planning, structure and content of csrs, with tips and examples from the niche medical writing team. Web the clinical study report (csr) is arguably the most important document emerging from a clinical trial. This document covers the structure and content of clinical study reports, synopsis, ethics, study design, population, treatment, statistical plan, efficacy and safety evaluation. Web learn how to write effective clinical study reports (csrs) for regulatory submission in the eu, based on ich e3 and e6 guidelines. Web the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with both required and optional components. It includes signature pages from investigators confirming the accuracy of the report. Browse by category or search by keyword to download the resources you need. Web the report guide is a supplementary resource to help you write up your clinical trial report for a scientific journal. Web this document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. Web learn how to write a clinical study report (csr) that meets regulatory requirements and best practices. This workshop provides an introduction to csr structure, content, benefits, challenges, and examples. Web this document provides questions and answers on the ich e3 guideline, which is a guideline, not a template, for reporting clinical studies. Web the clinical study report described in this guideline is an “integrated” full report of an individual study of any therapeutic, prophylactic, or diagnostic agent (referred to herein as.Clinical Trial Report Template TEMPLATES EXAMPLE TEMPLATES EXAMPLE
Clinical Evaluation Report Template Mdr
PPT Clinical Study Report PowerPoint Presentation, free download ID
Free Clinical Trial Templates Smartsheet
Clinical Research Report Synopsis Templates at
Clinical Study Protocol (CSP) Template Clinical Study Templates
Medical Report 10+ Examples, Format, Pdf, Tips
Clinical Study Report (CSR) Template Clinical Study Templates
Clinical Summary Template Fill Online, Printable, Fillable, Blank
Clinical Study Report Example in 2020 Templates, Professional
Learn About Topics Such As Clinical Study.
Web This Document Provides A Core Template For Clinical Study Reports That Can Be Used In Different Regions Of The World.
It Covers Topics Such As Content And Structure, Synopsis, Appendices, And Terminology Of Clinical Study Reports.
Web Core Reference Is A Guide For Medical Writers To Create Clinical Study Report (Csr) Content Based On Relevant Guidelines And Best Practices.
Related Post: