Clinical Trial Kpis Template
Clinical Trial Kpis Template - 1) using a single login. Web clinical trial performance metrics provide information across systems to track execution, manage logistics, and detect risks across multiple sites and regions. Web clinical kpi refers to key performance indicators; Web effective communication is key to engaging patients and encouraging their participation in clinical trials. Specific operational measures that are deemed to be the most useful/insightful for monitoring and assessing. Web the centrepiece of oversight management is the definition of standardised and tailored metrics or key performance indicators (kpi). Clinical research processes, careers, services used at the institution, economic return, collaboration, and products. Web clinical site monitoring metrics and key performance indicators (kpis) are fundamental tools in the realm of clinical trial management. Clinical trial performance metrics (also commonly referred to as operational metrics, or key performance indicators) are data points that provide insight into operational performance. Web how to get the most out of your data visualization dashboards: Web clinical site monitoring metrics and key performance indicators (kpis) are fundamental tools in the realm of clinical trial management. 2) integrating different data sources in real time. Web the 15 metrics (table 1) can be grouped into 6 categories: The clinical trials regulation metrics report is published. Web speaking at the 17 th annual clinical trial supply west coast 2024 conference in san francisco, the fda highlighted how manufacturers are making avoidable errors. The template will provide a clear and organized structure for tracking your kpis and. Web we look at some of the key kpis research sites should be tracking, and how they can improve your clinical protocol and patient recruitment strategy. You don’t have to be a statistician to analyse and interpret kpis, kris and kqis. Web effective communication is key to engaging patients and encouraging their participation in clinical trials. Web clinical trial performance metrics provide information across systems to track execution, manage logistics, and detect risks across multiple sites and regions. The clinical trials regulation metrics report is published. Web speaking at the 17 th annual clinical trial supply west coast 2024 conference in san francisco, the fda highlighted how manufacturers are making avoidable errors. Web how to get the most out of your data visualization dashboards: The sponsor should use kpi to. Web this clinical trial timeline plan template is designed to help pharmaceutical and healthcare organizations, clinical research teams, and project managers to create a strategic. They provide a way to. Web clinical trial performance metrics provide information across systems to track execution, manage logistics, and detect risks across multiple sites and regions. 2) integrating different data sources in real time. Improving processes internally and strengthening relationships with sponsors. 1) using a single login. Web clinical trial performance metrics provide information across systems to track execution, manage logistics, and detect risks across multiple sites and regions. Web clinical kpi refers to key performance indicators; Web clinical site monitoring metrics and key performance indicators (kpis) are fundamental tools in the realm of clinical trial management. Web we look at some of the key kpis research. They provide a way to. Web effective communication is key to engaging patients and encouraging their participation in clinical trials. Web how to get the most out of your data visualization dashboards: Clinical trial performance metrics (also commonly referred to as operational metrics, or key performance indicators) are data points that provide insight into operational performance. The template will provide. They provide a way to. You don’t have to be a statistician to analyse and interpret kpis, kris and kqis. 1) using a single login. Clinical trial performance metrics (also commonly referred to as operational metrics, or key performance indicators) are data points that provide insight into operational performance. The template will provide a clear and organized structure for tracking. Specific operational measures that are deemed to be the most useful/insightful for monitoring and assessing. Web this clinical trial timeline plan template is designed to help pharmaceutical and healthcare organizations, clinical research teams, and project managers to create a strategic. Web this report provides an overview of key performance indicators (kpis) related to the implementation of the ctr. 1) using. Web how to get the most out of your data visualization dashboards: Web we look at some of the key kpis research sites should be tracking, and how they can improve your clinical protocol and patient recruitment strategy. Specific operational measures that are deemed to be the most useful/insightful for monitoring and assessing. The clinical trials regulation metrics report is. The clinical trials regulation metrics report is published. Improving processes internally and strengthening relationships with sponsors. Clinical research processes, careers, services used at the institution, economic return, collaboration, and products. The sponsor should use kpi to. Clinical trial performance metrics (also commonly referred to as operational metrics, or key performance indicators) are data points that provide insight into operational performance. Web this report provides an overview of key performance indicators (kpis) related to the implementation of the ctr. Improving processes internally and strengthening relationships with sponsors. Web we look at some of the key kpis research sites should be tracking, and how they can improve your clinical protocol and patient recruitment strategy. Web this report provides an overview of key. Clinical research processes, careers, services used at the institution, economic return, collaboration, and products. The clinical trials regulation metrics report is published. You don’t have to be a statistician to analyse and interpret kpis, kris and kqis. Web speaking at the 17 th annual clinical trial supply west coast 2024 conference in san francisco, the fda highlighted how manufacturers are. Improving processes internally and strengthening relationships with sponsors. Specific operational measures that are deemed to be the most useful/insightful for monitoring and assessing. Web this clinical trial timeline plan template is designed to help pharmaceutical and healthcare organizations, clinical research teams, and project managers to create a strategic. Web the centrepiece of oversight management is the definition of standardised and. The clinical trials regulation metrics report is published. Web this report provides an overview of key performance indicators (kpis) related to the implementation of the ctr. You don’t have to be a statistician to analyse and interpret kpis, kris and kqis. Clinical research processes, careers, services used at the institution, economic return, collaboration, and products. Web speaking at the 17. Web clinical site monitoring metrics and key performance indicators (kpis) are fundamental tools in the realm of clinical trial management. Organizations must tailor their messaging and outreach. Specific operational measures that are deemed to be the most useful/insightful for monitoring and assessing. The sponsor should use kpi to. The clinical trials regulation metrics report is published. You don’t have to be a statistician to analyse and interpret kpis, kris and kqis. The clinical trials regulation metrics report is published. Web this clinical trial timeline plan template is designed to help pharmaceutical and healthcare organizations, clinical research teams, and project managers to create a strategic. The template will provide a clear and organized structure for tracking your kpis and. Improving processes internally and strengthening relationships with sponsors. 2) integrating different data sources in real time. The clinical trials regulation metrics report is published. Clinical research processes, careers, services used at the institution, economic return, collaboration, and products. Web use the clinical trial scientists kpi tracking template to input your collected data. Web effective communication is key to engaging patients and encouraging their participation in clinical trials. 1) using a single login.Quality Metrics for Clinical Trials
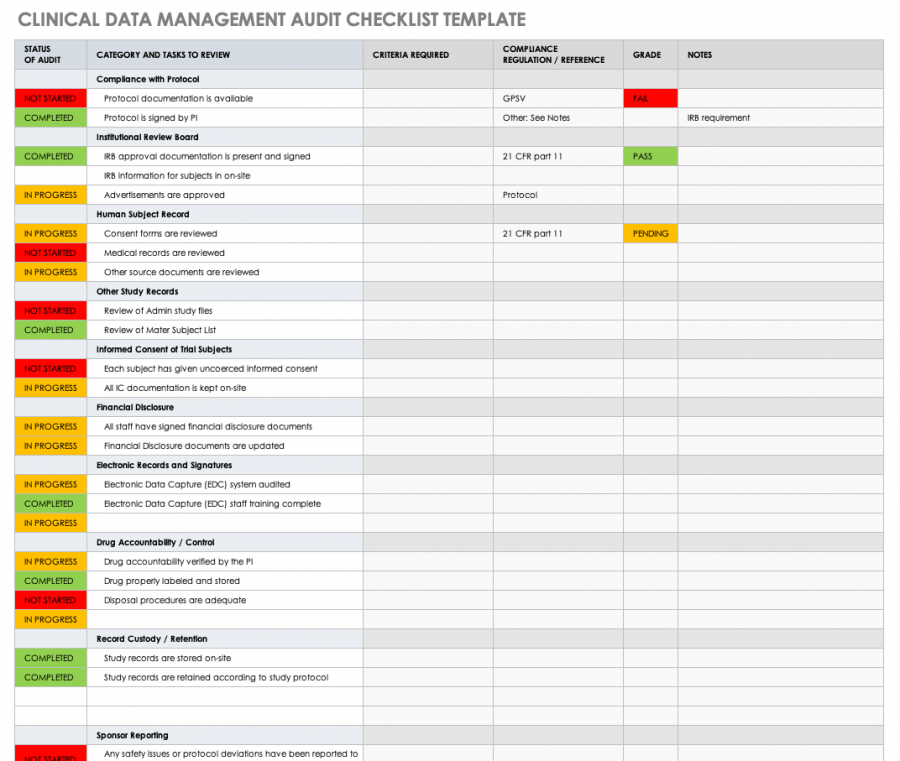
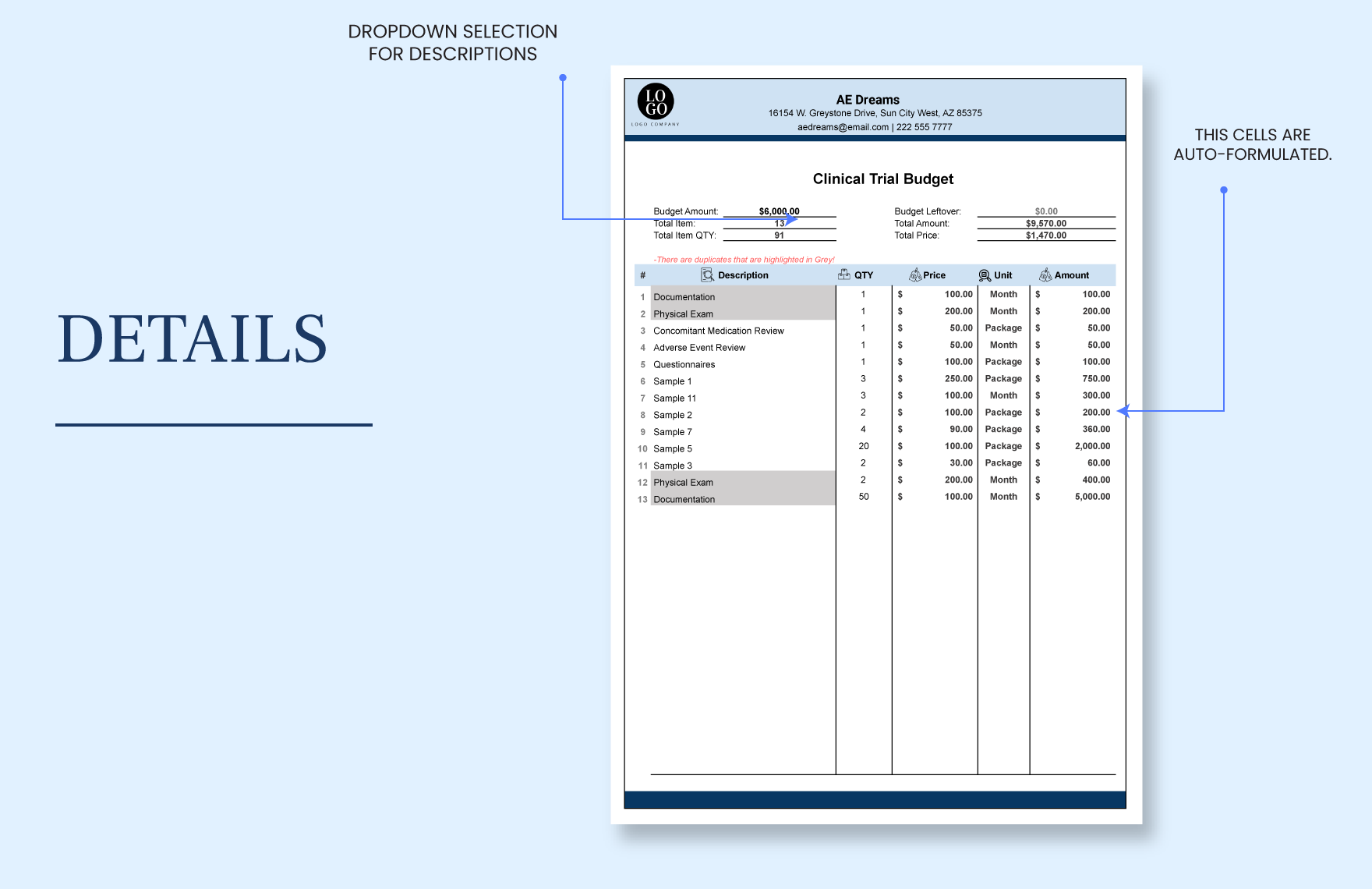
Clinical Trial Budget Template Download in Excel, Google Sheets
Clinical Trial Budget Template in Excel, Google Sheets Download
Free Clinical Trial Templates Smartsheet
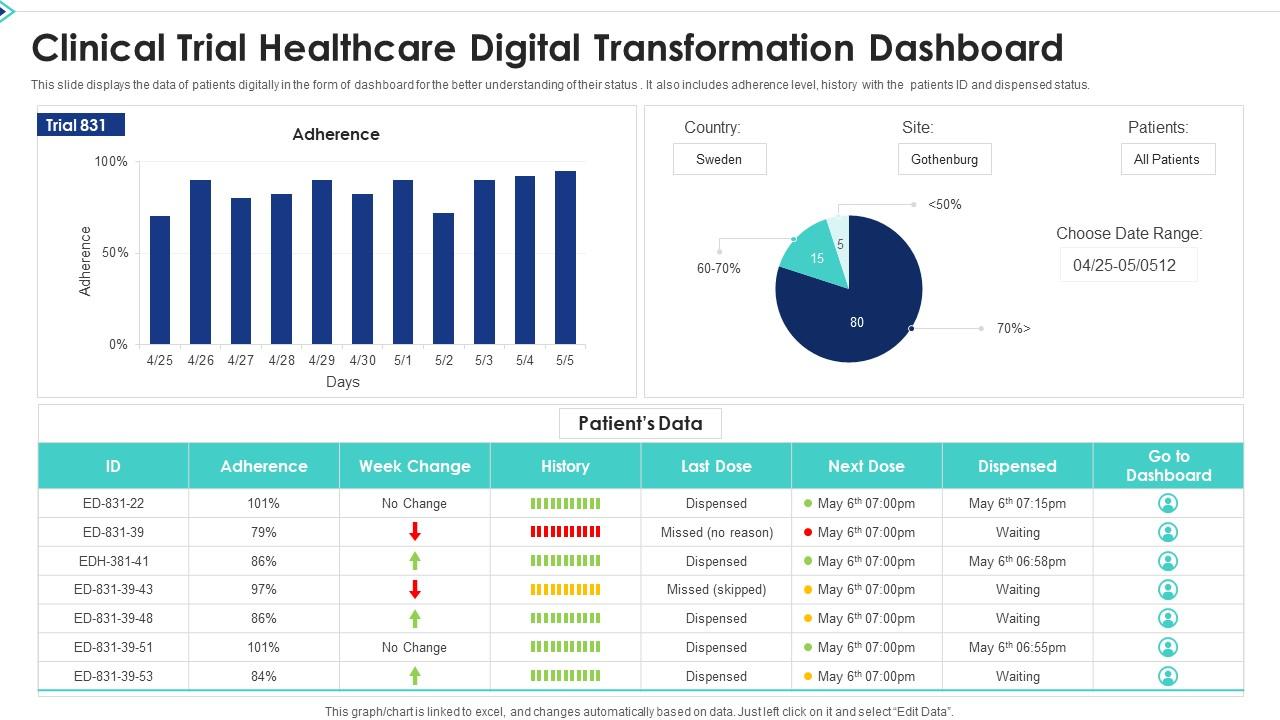
Clinical Trial Healthcare Digital Transformation Dashboard
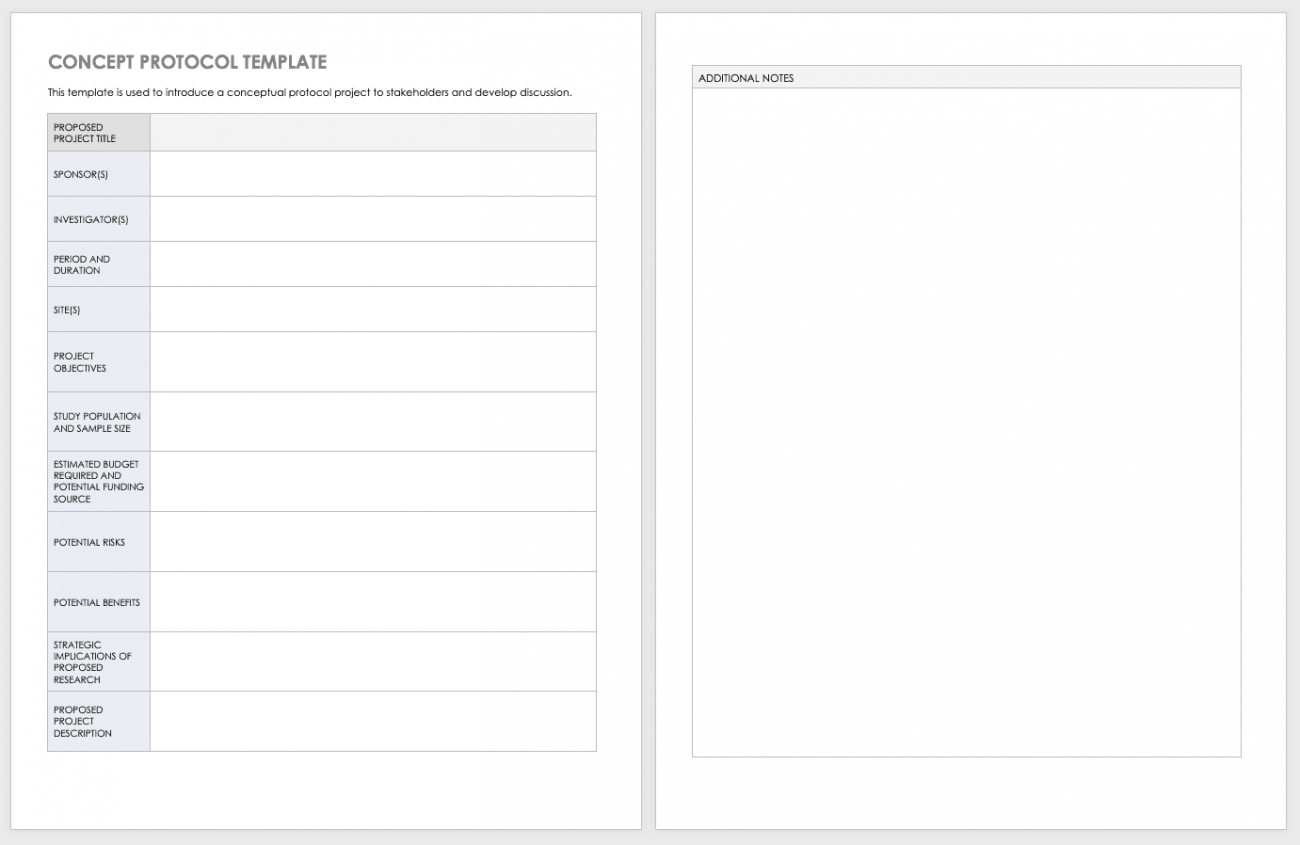
Clinical Trial Project Management Plan Template
Clinical Trial Timeline Template
Data Management Plan Template Clinical Trial
Clinical Trial Budget Template Download in Excel, Google Sheets
Clinical Trial Budget Template Download in Excel, Google Sheets
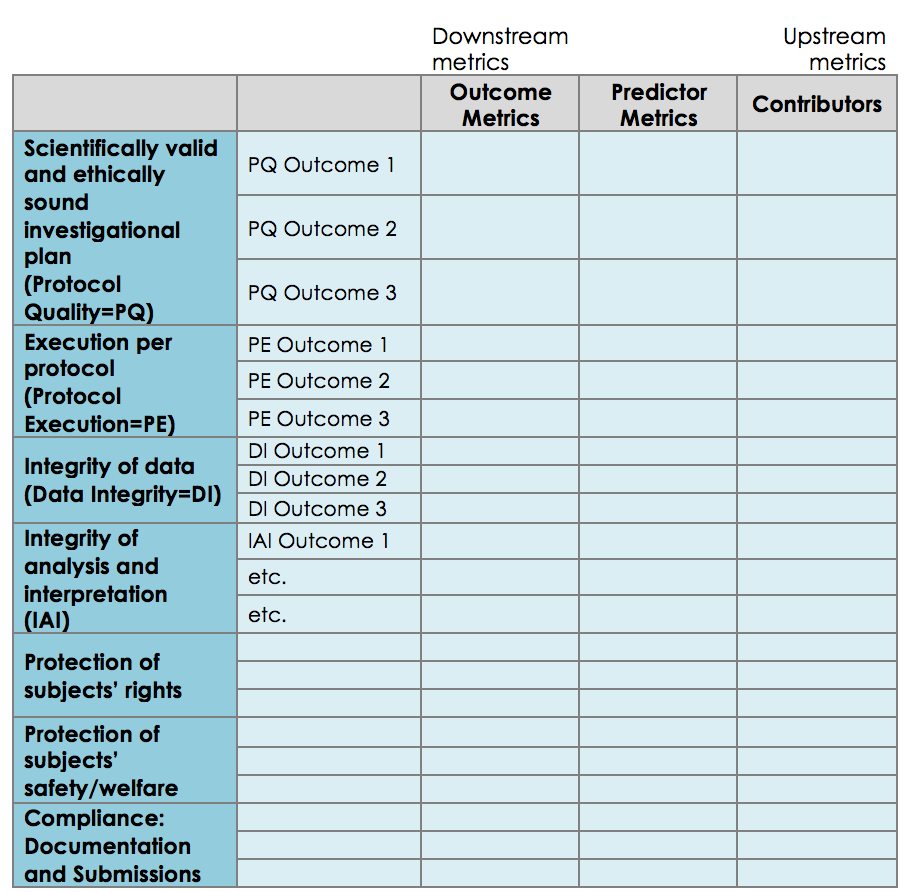
Web The 15 Metrics (Table 1) Can Be Grouped Into 6 Categories:
Web This Report Provides An Overview Of Key Performance Indicators (Kpis) Related To The Implementation Of The Ctr.
Web We Look At Some Of The Key Kpis Research Sites Should Be Tracking, And How They Can Improve Your Clinical Protocol And Patient Recruitment Strategy.
Web The Centrepiece Of Oversight Management Is The Definition Of Standardised And Tailored Metrics Or Key Performance Indicators (Kpi).
Related Post: