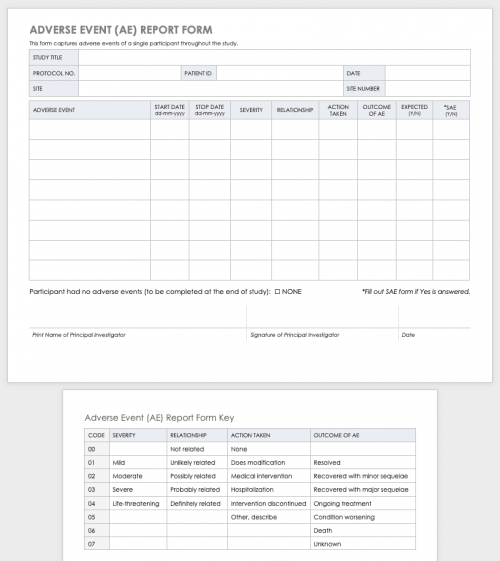
Clinical Trial Report Template
Clinical Trial Report Template - Having reached step 4 of the ich process at the ich steering. Web structure and content of clinical study reports. Please note that this page has been updated for 2015 following a quality check and review of the templates, and. Web the common clinical study report (csr) template provides a common, streamlined structure to report data, adhere to global requirements, enable disclosures, and. Web welcome to global health trials' tools and templates library. Web fda releases draft guidance on oncology multiregional clinical trials. Web protocol templates for clinical trials. Web this clinical study report (csr) template is specifically designed to be used in conjunction with the clinical study protocol (csp) template. Web the objective of this guideline is to facilitate the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Web consequently, many organisations involved in conducting clinical trials employ their own csr templates, sometimes with associated guidance documents that describe how the. Web welcome to global health trials' tools and templates library. Web the objective of this guideline is to facilitate the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Having reached step 4 of the ich process at the ich steering. Web protocol templates for clinical trials. Ich e3 offers a csr template to guide you in terms of providing the proper data and content in a specified order and format. This guideline can be found. Web fda releases draft guidance on oncology multiregional clinical trials. Web the clinical study report described in this guideline is an integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein. Please note that this page has been updated for 2015 following a quality check and review of the templates, and. Web structure and content of clinical study reports. Web welcome to global health trials' tools and templates library. Web protocol templates for clinical trials. Web learn how to prepare tables, listings, and figures for clinical study report and submit to regulatory agencies, the essential part of clinical trial development. This guideline can be found. Web the clinical study report described in this guideline is an integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein. Web structure and content of clinical study reports. Please note that this page has been updated for 2015 following a quality check and review of the templates, and. Web this document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Web this clinical study report (csr) template is specifically designed to be used in conjunction with the clinical study protocol (csp) template. Web topics included in the report guide cover reporting checklists, trial report structure, choice of title, writing style, trial registry and reporting consistency, spin or reporting. Web learn how to prepare tables, listings, and figures for clinical study report and submit to regulatory agencies, the essential part of clinical trial development. Web the common clinical study report (csr) template provides a common, streamlined structure to report data, adhere to global requirements, enable disclosures, and. Web this clinical study report (csr) template is specifically designed to be. Web the common clinical study report (csr) template provides a common, streamlined structure to report data, adhere to global requirements, enable disclosures, and. Having reached step 4 of the ich process at the ich steering. Web this clinical study report (csr) template is specifically designed to be used in conjunction with the clinical study protocol (csp) template. Web structure and. Web the clinical study report described in this guideline is an integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein. Ich e3 offers a csr template to guide you in terms of providing the proper data and content in a specified order and format. Web fda releases draft guidance on oncology multiregional. Web topics included in the report guide cover reporting checklists, trial report structure, choice of title, writing style, trial registry and reporting consistency, spin or reporting. Trishula therapeutics reports results from phase i trial of pancreatic cancer drug. Web protocol templates for clinical trials. Web unlock the key to effective clinical trial reporting through medical writing. Web the clinical study. Trishula therapeutics reports results from phase i trial of pancreatic cancer drug. Phase 2 or 3 clinical trials that. Web consequently, many organisations involved in conducting clinical trials employ their own csr templates, sometimes with associated guidance documents that describe how the. Web learn how to prepare tables, listings, and figures for clinical study report and submit to regulatory agencies,. Web the clinical study report described in this guideline is an integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein. This template aims to facilitate the development of phase. Web structure and content of clinical study reports. Phase 2 or 3 clinical trials that. Learn the essentials in our comprehensive guide. Ich e3 offers a csr template to guide you in terms of providing the proper data and content in a specified order and format. Web unlock the key to effective clinical trial reporting through medical writing. Web template for writing a clinical study report of clinical trial conducted under chapter 4 other clinical trials, of the clinical trial ordinance (clino).. Having reached step 4 of the ich process at the ich steering. Web template for writing a clinical study report of clinical trial conducted under chapter 4 other clinical trials, of the clinical trial ordinance (clino). Web unlock the key to effective clinical trial reporting through medical writing. Web welcome to global health trials' tools and templates library. This guideline. Web the clinical study report described in this guideline is an integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein. Web unlock the key to effective clinical trial reporting through medical writing. Web the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with. This. Phase 2 or 3 clinical trials that. Web this document aims to allow the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Learn the essentials in our comprehensive guide. Web welcome to global health trials' tools and templates library. Web learn how to prepare tables, listings, and figures for clinical study. Web the objective of this guideline is to facilitate the compilation of a single core clinical study report acceptable to all regulatory authorities of the ich regions. Web this clinical study report (csr) template is specifically designed to be used in conjunction with the clinical study protocol (csp) template. Web unlock the key to effective clinical trial reporting through medical writing. This guideline can be found. Web welcome to global health trials' tools and templates library. Web protocol templates for clinical trials. Web the clinical study report described in this guideline is an integrated full report of an individual study of any therapeutic, prophylactic or diagnostic agent (referred to herein. The templates below have been shared by other groups, and are free to use and adapt for your research studies. Web the ich m11 clinical electronic structured harmonised protocol template provides comprehensive clinical protocol organization with standardized content with. Web fda releases draft guidance on oncology multiregional clinical trials. Learn the essentials in our comprehensive guide. Having reached step 4 of the ich process at the ich steering. Web nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: Phase 2 or 3 clinical trials that. Web structure and content of clinical study reports. Web welcome to global health trials' tools and templates library.Trial Report Template PROFESSIONAL TEMPLATES PROFESSIONAL TEMPLATES
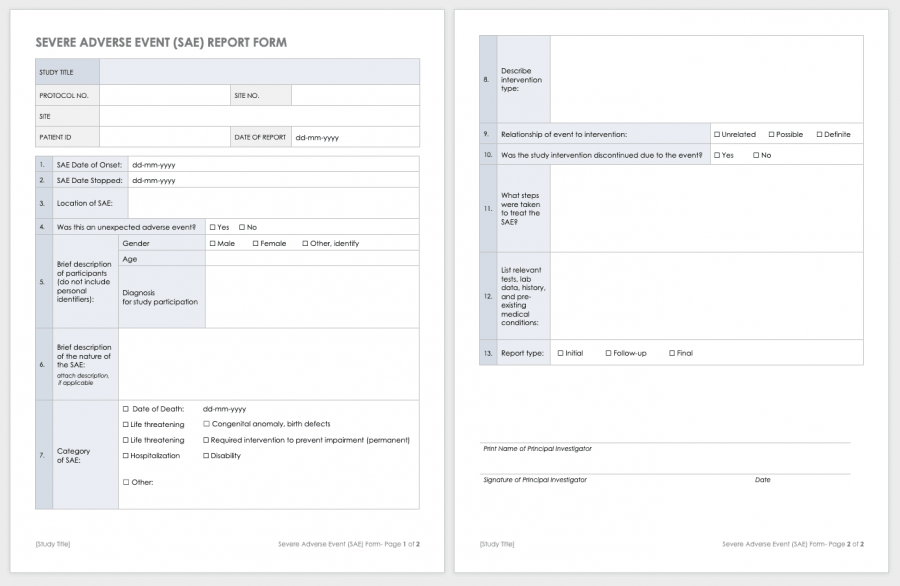
Free Clinical Trial Templates Smartsheet
Clinical Trial Report Template TEMPLATES EXAMPLE TEMPLATES EXAMPLE
Free Clinical Trial Templates Smartsheet
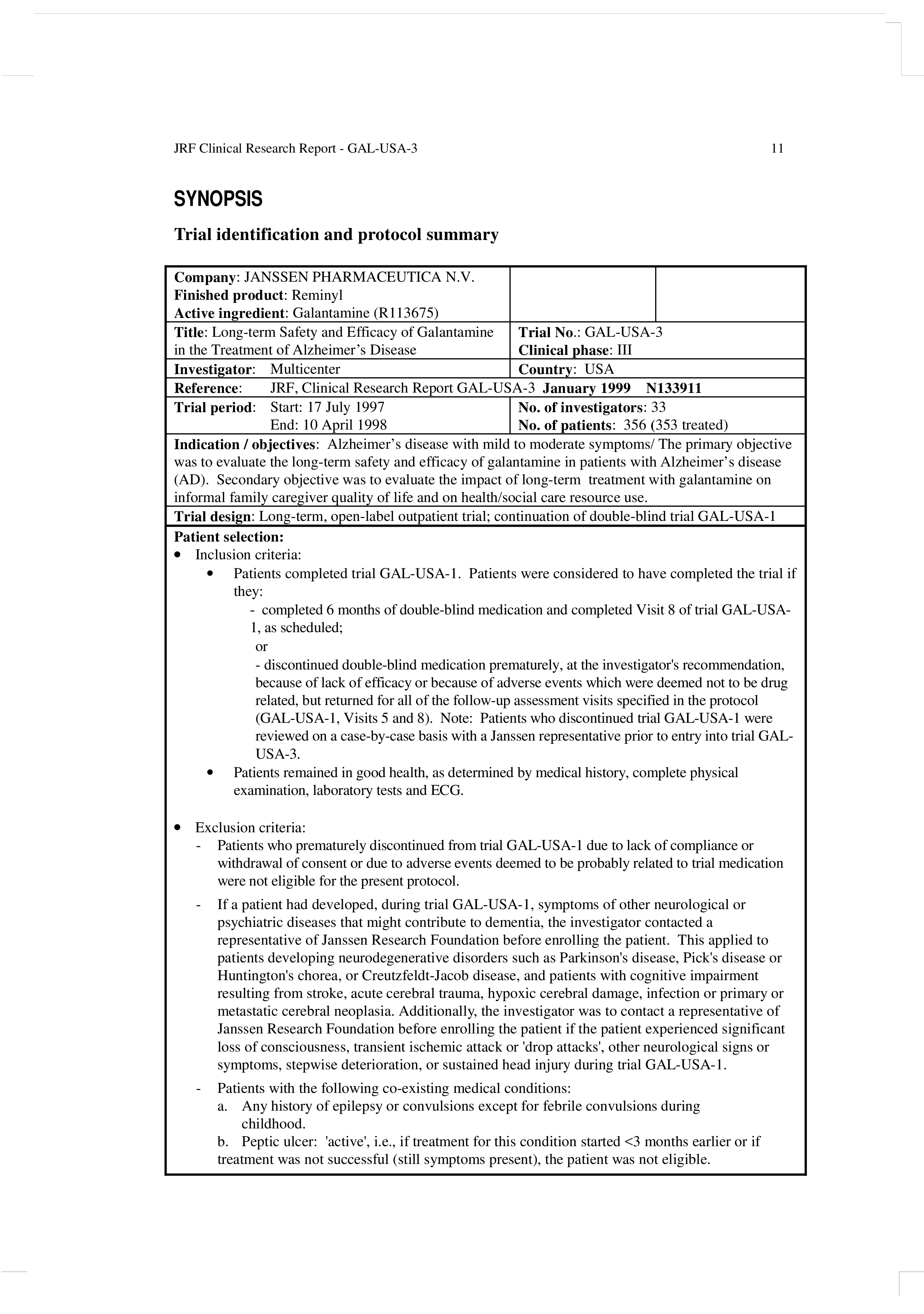
Clinical Research Report Synopsis Templates at
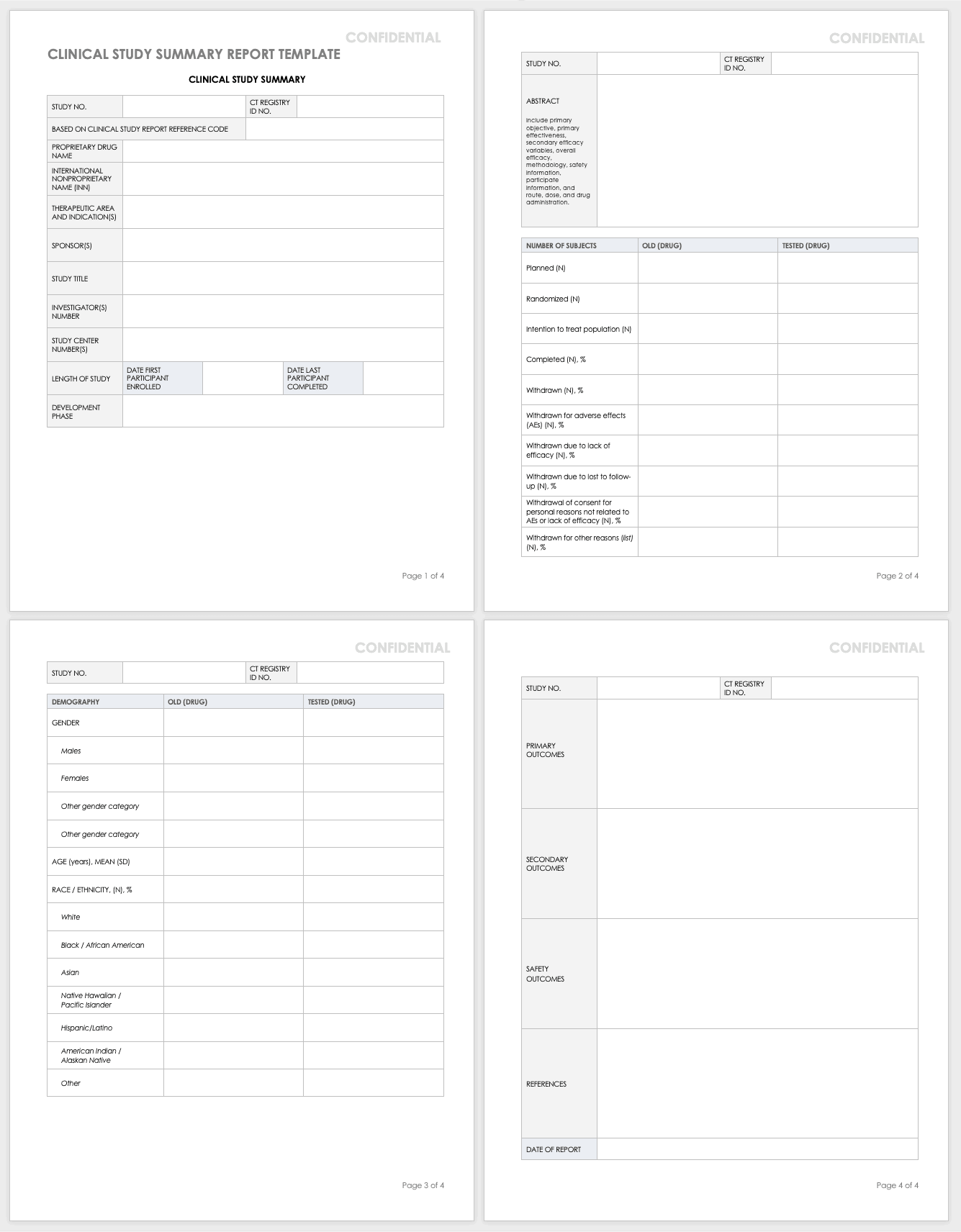
Free Clinical Trial Templates Smartsheet
Clinical Trial Report Template (3) TEMPLATES EXAMPLE TEMPLATES
Clinical Trial Report Template (3) PROFESSIONAL TEMPLATES
Free Clinical Trial Templates Smartsheet
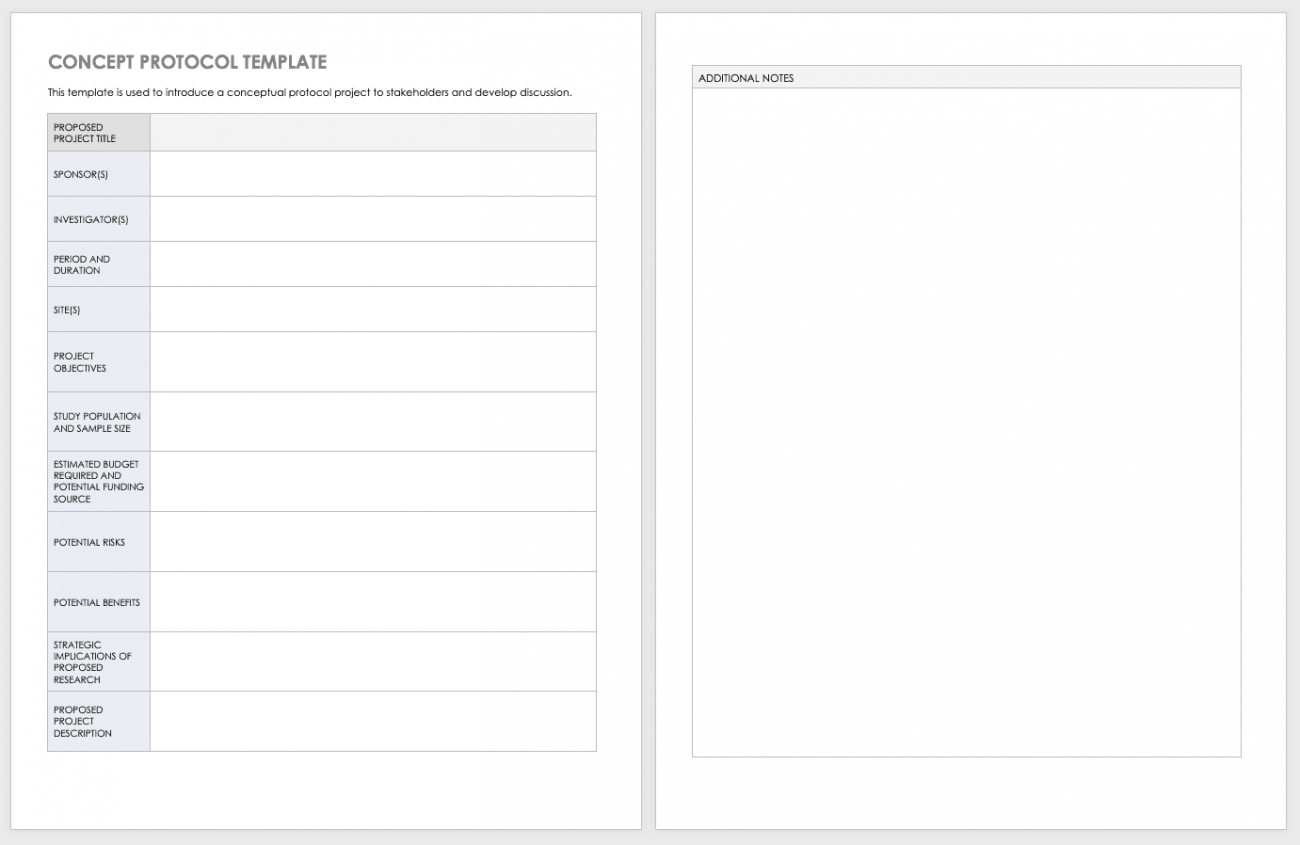
Free Clinical Trial Templates Smartsheet In Trial Report Template
Web Template For Writing A Clinical Study Report Of Clinical Trial Conducted Under Chapter 4 Other Clinical Trials, Of The Clinical Trial Ordinance (Clino).
Ich E3 Offers A Csr Template To Guide You In Terms Of Providing The Proper Data And Content In A Specified Order And Format.
Please Note That This Page Has Been Updated For 2015 Following A Quality Check And Review Of The Templates, And.
Web Topics Included In The Report Guide Cover Reporting Checklists, Trial Report Structure, Choice Of Title, Writing Style, Trial Registry And Reporting Consistency, Spin Or Reporting.
Related Post: