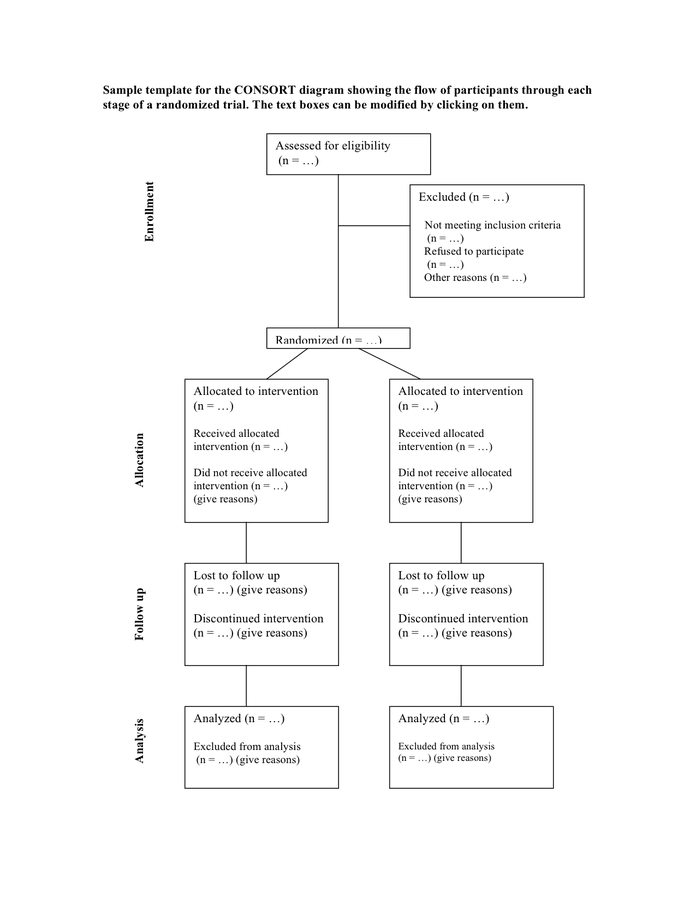
Consort Flow Diagram Template
Consort Flow Diagram Template - The development of consort guidelines has received considerable international recognition. It provides guidance for reporting all randomised controlled trials, but focuses on the most common design type—individually randomised, two group, parallel trials. Of 469 primary reports of. We assessed the proportion of parallel group trial publications reporting specific items recommended by consort for inclusion in a flow diagram. Web the consort 2010 statement is this paper including the 25 item checklist in the table ⇓ and the flow diagram ⇓. Web the flow diagram included in each rct was assessed for completeness of reporting in relation to published criteria and the consort flow diagram template. The flowchart is meant to conform to the specifications of the consort 2010 statement guidelines adhered to by the majority of scientific biomedical. Web several examples of flow diagrams are included. The statement facilitates critical appraisal and interpretation of rcts. Analysed n = excluded from analysis n = give reasons n =. It provides guidance for reporting all randomised controlled trials, but focuses on the most common design type—individually randomised, two group, parallel trials. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: Web the flow diagram included in each rct was assessed for completeness of reporting in relation to published criteria and the consort flow diagram template. It provides guidance for reporting all randomised controlled trials, but focuses on the most common design type—individually randomised, two group, parallel trials. The flowchart is meant to conform to the specifications of the consort 2010 statement guidelines adhered to by the majority of scientific biomedical. Web sample template for the consort diagram showing the flow of participants through each stage of a randomized trial. Web a diagram showing the flow of participants through a trial is strongly recommended by consort; Web the consort statement comprises a checklist of the minimum essential items that should be included in reports of randomised trials and a diagram documenting the flow of participants through the trial. Web this example shows how to use the pgf/tikz package within a latex article class document to make a flowchart of participants progress through the phases of a randomized controlled trial. Many leading medical journals and major international editorial groups have endorsed the consort statement. It provides guidance for reporting all randomised controlled trials, but focuses on the most common design type—individually randomised, two group, parallel trials. Web the consort statement comprises a checklist of the minimum essential items that should be included in reports of randomised trials and a diagram documenting the flow of participants through the trial. Analysed n = excluded from analysis n = give reasons n =. Web the statement consists of a checklist and flow diagram that authors can use for reporting an rct. The suggested template is shown in fig. It provides guidance for reporting all randomised controlled trials, but focuses on the most common design type—individually randomised, two group, parallel trials. Web the consort 2010 statement is this paper including the 25 item checklist in the table ⇓ and the flow diagram ⇓. Web this example shows how to use the pgf/tikz package within a latex article class document to make a flowchart of participants progress through the phases of a randomized controlled trial. The flowchart is meant to conform to the specifications of the consort 2010 statement guidelines adhered to by the majority of scientific biomedical. Web a cross sectional review of all primary reports of randomized trials which included a consort flow diagram indexed in pubmed core clinical journals (2009). Web the flow diagram included in each rct was assessed for completeness of reporting in relation to published criteria and the consort flow diagram template. Web it presents the meaning and rationale for each new and updated checklist item providing examples of good reporting and, where possible, references to relevant empirical studies. Reporting guidelines for clinical trial reports for interventions. Allocated to intervention n = received intervention n = did not receive intervention n = give reasons n =. Several examples of flow diagrams are included. Web the consort 2010 statement is this paper including the 25 item checklist in the table (table 1) and the flow diagram (figure 1). The flowchart is meant to conform to the specifications of. Web the expanded consort figure draws on theory, a prior meeting and recent recommendations for reporting factors related to external validity. The layout of a consort diagram depends on the study design. Web the statement consists of a checklist and flow diagram that authors can use for reporting an rct. Web several examples of flow diagrams are included. It provides. Many leading medical journals and major international editorial groups have endorsed the consort statement. Allocated to intervention n = received intervention n = did not receive intervention n = give reasons n =. A consort diagram can be generated by either visual basic scripting (vbs), sas/graph using annotate macros or Web the consort 2010 statement is this paper including the. The flowchart is meant to conform to the specifications of the consort 2010 statement guidelines adhered to by the majority of scientific biomedical. Web the consort statement comprises a checklist of the minimum essential items that should be included in reports of randomised trials and a diagram documenting the flow of participants through the trial. Several examples of flow diagrams. = give reasons n =. Liu x, rivera sc, moher d, calvert mj, denniston ak; We assessed the proportion of parallel group trial publications reporting specific items recommended by consort for inclusion in a flow diagram. Bmj publishing group limited (bmj) disclaims all liability and responsibility arising from any reliance placed on this supplemental material which has been supplied by. Web the flow diagram included in each rct was assessed for completeness of reporting in relation to published criteria and the consort flow diagram template. Several examples of flow diagrams are included. = give reasons n =. Web several examples of flow diagrams are included. Several examples of flow diagrams are included. Web it presents the meaning and rationale for each new and updated checklist item providing examples of good reporting and, where possible, references to relevant empirical studies. It expands the basic consort flow diagram for clinical trials to summarize external validity and contextual factors more concisely and transparently. Many leading medical journals and major international editorial groups have endorsed the. Many leading medical journals and major international editorial groups have endorsed the consort statement. A consort diagram can be generated by either visual basic scripting (vbs), sas/graph using annotate macros or Liu x, rivera sc, moher d, calvert mj, denniston ak; It expands the basic consort flow diagram for clinical trials to summarize external validity and contextual factors more concisely. 50.9 kb ) for free. Web the statement consists of a checklist and flow diagram that authors can use for reporting an rct. The suggested template is shown in fig. Bmj publishing group limited (bmj) disclaims all liability and responsibility arising from any reliance placed on this supplemental material which has been supplied by the author(s) sex transm infect. The. Web the consort statement comprises a checklist of the minimum essential items that should be included in reports of randomised trials and a diagram documenting the flow of participants through the trial. 50.9 kb ) for free. The statement facilitates critical appraisal and interpretation of rcts. Allocated to intervention n = received intervention n = did not receive intervention n = give reasons n =. The development of consort guidelines has received considerable international recognition. The exact form and content of the flow diagram may vary according to specific features of a trial. The flowchart is meant to conform to the specifications of the consort 2010 statement guidelines adhered to by the majority of scientific biomedical. Web sample template for the consort diagram showing the flow of participants through each stage of a randomized trial. Web several examples of flow diagrams are included. = give reasons n =. It provides guidance for reporting all randomised controlled trials, but focuses on the most common design type—individually randomised, two group, parallel trials. Web the consort 2010 statement is this paper including the 25 item checklist in the table (table 1) and the flow diagram (figure 1). Web download or preview 1 pages of pdf version of sample template for the consort diagram (doc: Web the consort 2010 statement is this paper including the 25 item checklist in the table ⇓ and the flow diagram ⇓. Web it presents the meaning and rationale for each new and updated checklist item providing examples of good reporting and, where possible, references to relevant empirical studies. Web this example shows how to use the pgf/tikz package within a latex article class document to make a flowchart of participants progress through the phases of a randomized controlled trial.Consort Flow Diagram Template Word
Consort Flow Diagram Template
Sample template for the consort diagram in Word and Pdf formats
Sample template for the consort diagram in Word and Pdf formats
Consort Flow Chart Example Flowchart Examples
Consort Flow Chart Template
Consort Flow Chart Template
Consort Flow Diagram Template
Consort Flow Diagram Template
CONSORT 2010 flow diagram. Download Scientific Diagram
Several Examples Of Flow Diagrams Are Included.
The Layout Of A Consort Diagram Depends On The Study Design.
Several Examples Of Flow Diagrams Are Included.
Liu X, Rivera Sc, Moher D, Calvert Mj, Denniston Ak;
Related Post: