Free Decentralized Clinical Trials Staff Training Checklist Templates
Free Decentralized Clinical Trials Staff Training Checklist Templates - The right training is essential for helping you and your staff learn how to fully leverage the key benefits a modern ctms offers, such as the flexibility of user roles, group security, user. We aimed to provide an overview of the landscape of dcts by comparing regulatory guidance reports and analyzing decentralized elements from clinical trial registries. Web a resource outlining the steps needed to clear a path forward for decentralized clinical trial implementation Web decentralized clinical trials can be completely remote or partially decentralized with hybrid approaches. Web new clinical research staff orientation & training checklist template. Understand what needs to be considered and measured before selecting a digital health technology. Be sure each member of your current staff is trained before your ctms Track a detailed trial plan, centralize participant screening/enrollment, identify risks & issues, and help get your trial completed on time. Provides easy access for trial monitors, auditors, and regulatory authorities; Web manage your clinical trials pipeline in each phase. Though not legally binding, maintaining a. Be sure each member of your current staff is trained before your ctms Decentralized clinical trials (dcts), novel endpoints, stakeholder perceptions, and mobile technologies. Web clinical trials transformation initiative (ctti) initiated the mobile clinical trials (mct) program, which includes four projects focused on the following topics: Within each domain are relevant decentralized approaches that may be part of a dct and the questions for irb/ec review. Web new clinical research staff orientation & training checklist template. Web your approach to running a decentralized clinical trial doesn’t have to be all or nothing. Web welcome to global health trials' tools and templates library. Selecting & testing a digital health technology. New hire and onboarding resources, core training, and supplemental or advanced training. Decentralized clinical trials (dcts), novel endpoints, stakeholder perceptions, and mobile technologies. Web verify all staff members are trained for their role in the ctms. Though not legally binding, maintaining a. Track a detailed trial plan, centralize participant screening/enrollment, identify risks & issues, and help get your trial completed on time. Web patient recruitment and consent. The mct dct project concentrates on the actual and perceived legal, Be sure each member of your current staff is trained before your ctms Web this document is a checklist of the issues that irbs/ecs should consider when reviewing a decentralized clinical trial. Provides easy access for trial monitors, auditors, and regulatory authorities; The checklist is organized into three domains: Decentralized clinical trials (dcts), novel endpoints, stakeholder perceptions, and mobile technologies. Please consult the main clinical research toolkit page for information. New hire and onboarding resources, core training, and supplemental or advanced training. We aimed to provide an overview of the landscape of dcts by comparing regulatory guidance reports and analyzing decentralized elements from clinical trial registries. Web ucsf has. Web a resource outlining the steps needed to clear a path forward for decentralized clinical trial implementation Web a regulatory binder is essential for managing clinical trial documents, ensuring regulatory compliance, and facilitating audits. This resource has been removed. Understand what needs to be considered and measured before selecting a digital health technology. Web manage your clinical trials pipeline in. Provides easy access for trial monitors, auditors, and regulatory authorities; Web your approach to running a decentralized clinical trial doesn’t have to be all or nothing. New hire and onboarding resources, core training, and supplemental or advanced training. The checklist is organized into three domains: Selecting & testing a digital health technology. The checklist is organized into three domains: Web clinical trials transformation initiative (ctti) initiated the mobile clinical trials (mct) program, which includes four projects focused on the following topics: The mct dct project concentrates on the actual and perceived legal, Incorporate mobile research into the trial protocol at an early stage. Track a detailed trial plan, centralize participant screening/enrollment, identify. Hybrid trials are those that require some visits to be conducted on site, while other visits or assessments can be performed at a participant’s home or within their local care community. We aimed to provide an overview of the landscape of dcts by comparing regulatory guidance reports and analyzing decentralized elements from clinical trial registries. Please note that this page. Be sure each member of your current staff is trained before your ctms People, data collection, and remote data oversight. Decentralized clinical trials (dcts), novel endpoints, stakeholder perceptions, and mobile technologies. Please consult the main clinical research toolkit page for information. Web your approach to running a decentralized clinical trial doesn’t have to be all or nothing. Web a regulatory binder is essential for managing clinical trial documents, ensuring regulatory compliance, and facilitating audits. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones have been added. New hire and onboarding resources, core training, and supplemental or advanced training. Web a resource outlining the. Selecting & testing a digital health technology. Decentralized clinical trials (dcts), novel endpoints, stakeholder perceptions, and mobile technologies. New hire and onboarding resources, core training, and supplemental or advanced training. Hybrid trials are those that require some visits to be conducted on site, while other visits or assessments can be performed at a participant’s home or within their local care. Web effective training teaches researchers how to ethically and compliantly execute study activities, communicate with participants, manage regulatory requirements, interact with research tools and technology, and provide proper oversight of staff. Web advantages of incorporating decentralized attributes and how barriers to their inclusion can be overcome. Web your approach to running a decentralized clinical trial doesn’t have to be all. And serves as a reference for the research team. Incorporate mobile research into the trial protocol at an early stage. The right training is essential for helping you and your staff learn how to fully leverage the key benefits a modern ctms offers, such as the flexibility of user roles, group security, user. Web the following training checklist is based. Web new clinical research staff orientation & training checklist template. The right training is essential for helping you and your staff learn how to fully leverage the key benefits a modern ctms offers, such as the flexibility of user roles, group security, user. Incorporate mobile research into the trial protocol at an early stage. Web decentralized clinical trials can be completely remote or partially decentralized with hybrid approaches. Decentralized clinical trials (dcts), novel endpoints, stakeholder perceptions, and mobile technologies. Web this document is a checklist of the issues that irbs/ecs should consider when reviewing a decentralized clinical trial. Web the following checklist can help guide sponsors and cros toward a successful decentralized trial or effective integration of a hybrid approach. Track a detailed trial plan, centralize participant screening/enrollment, identify risks & issues, and help get your trial completed on time. Understand what needs to be considered and measured before selecting a digital health technology. Hybrid trials are those that require some visits to be conducted on site, while other visits or assessments can be performed at a participant’s home or within their local care community. Web patient recruitment and consent. Web ucsf has created a comprehensive checklist of training steps for clinical researchers, which breaks training activities apart into three categories: Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones have been added. Web your approach to running a decentralized clinical trial doesn’t have to be all or nothing. Web manage your clinical trials pipeline in each phase. The checklist is organized into three domains:Free Clinical Trial Templates Smartsheet
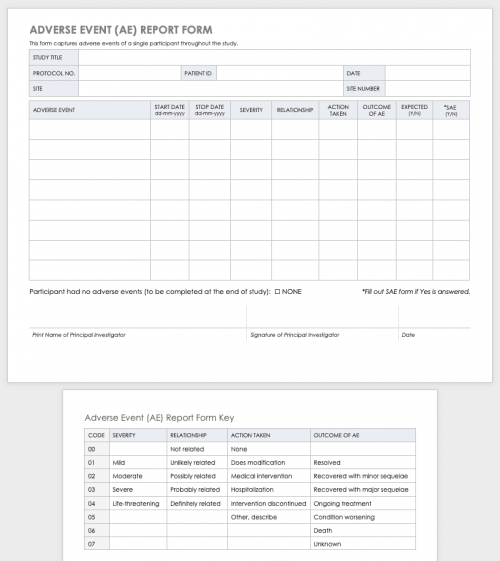
Free Clinical Trial Templates Smartsheet
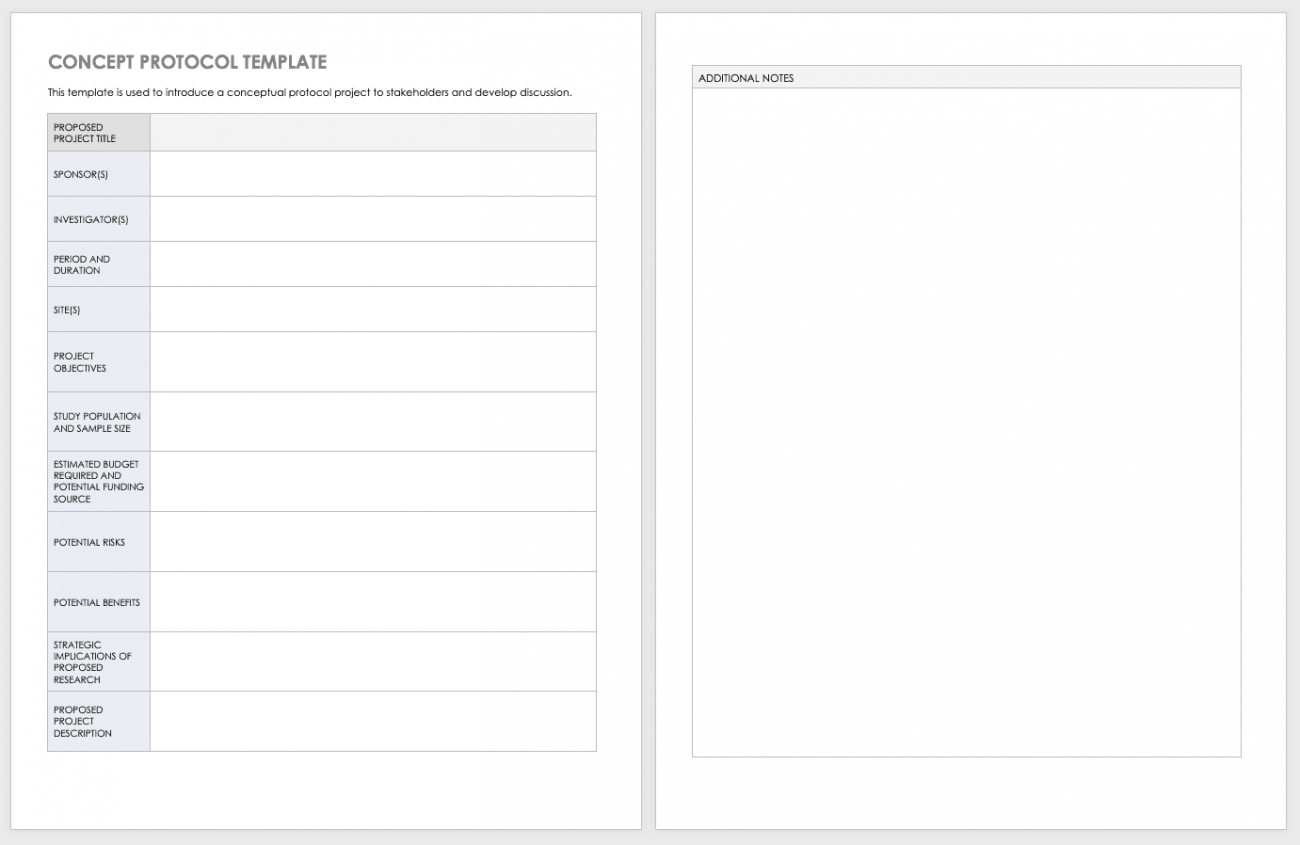
Free Clinical Trial Templates Smartsheet
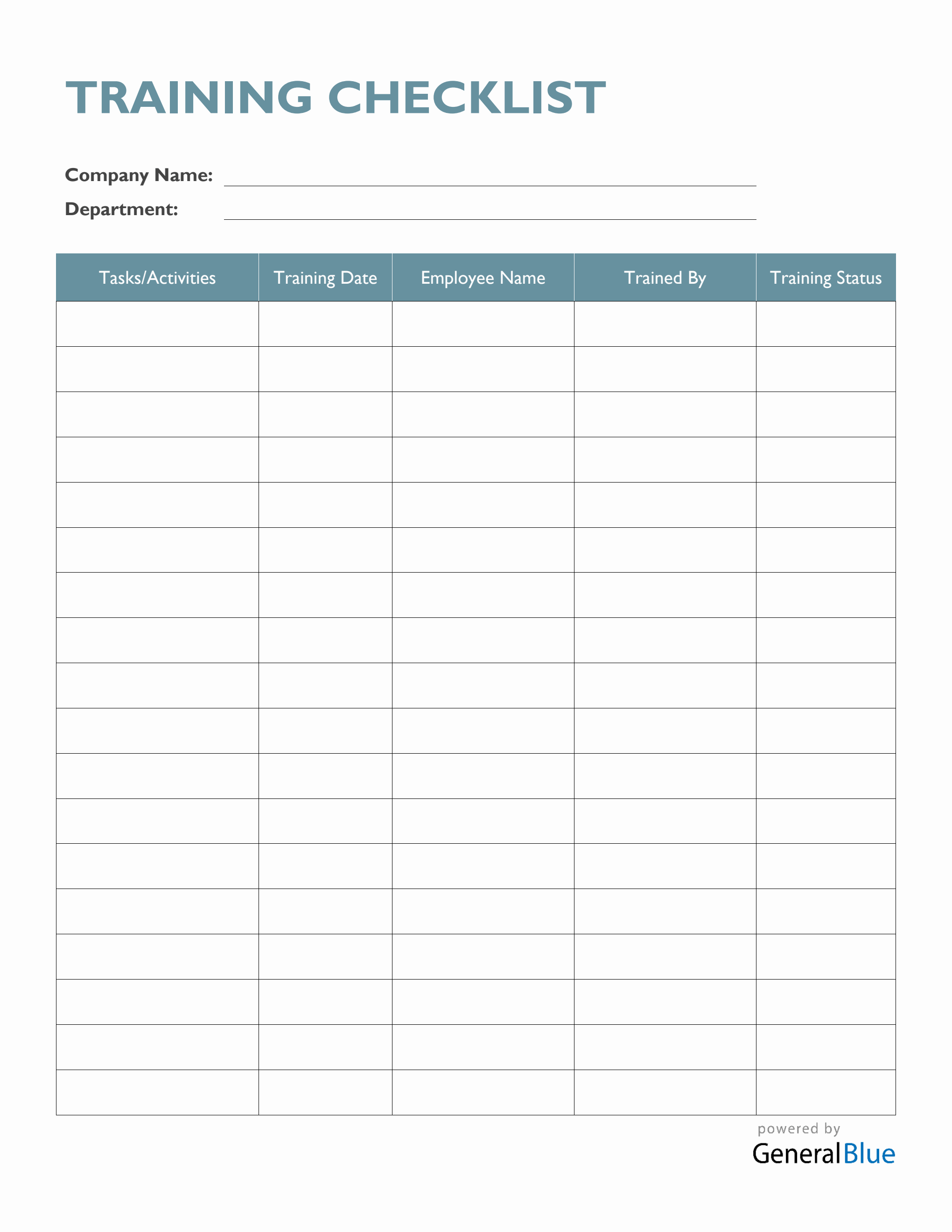
Training Checklist Template Word Free Start Your Free Trial Today
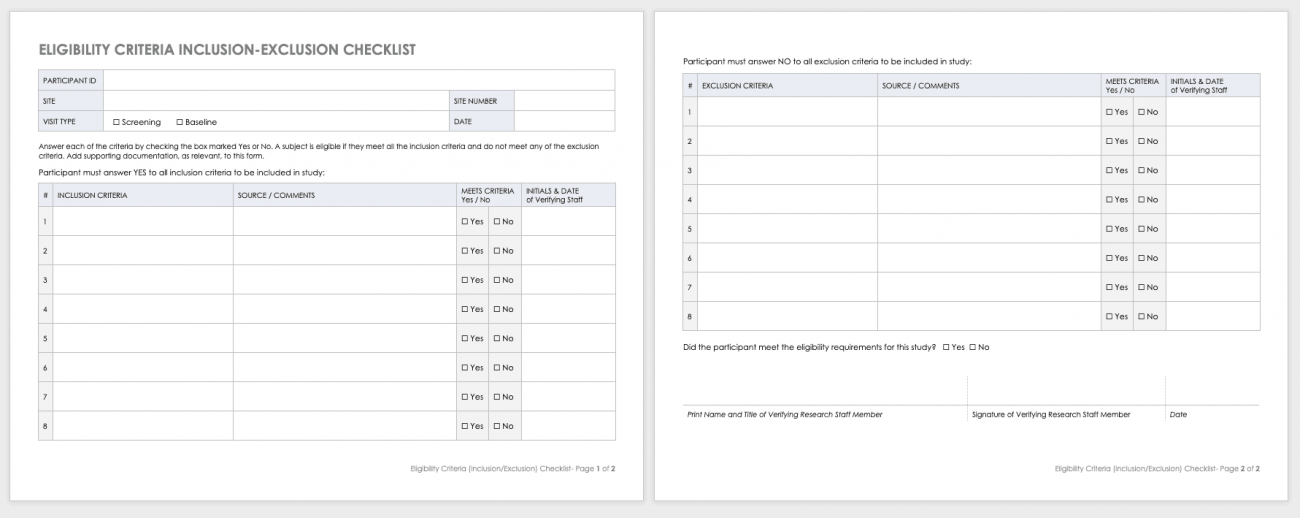
Free Clinical Trial Templates Smartsheet
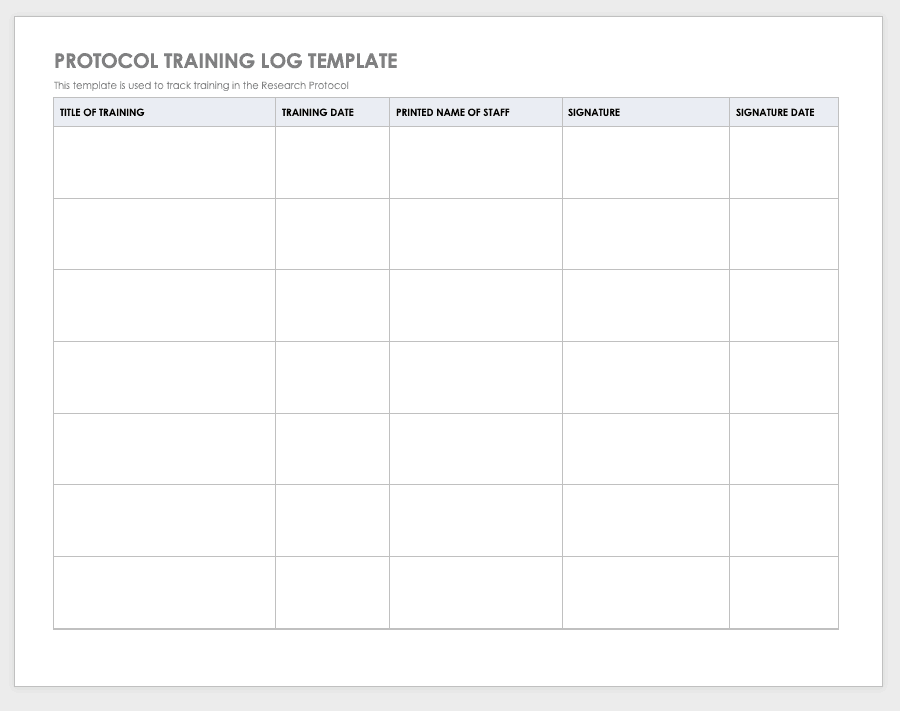
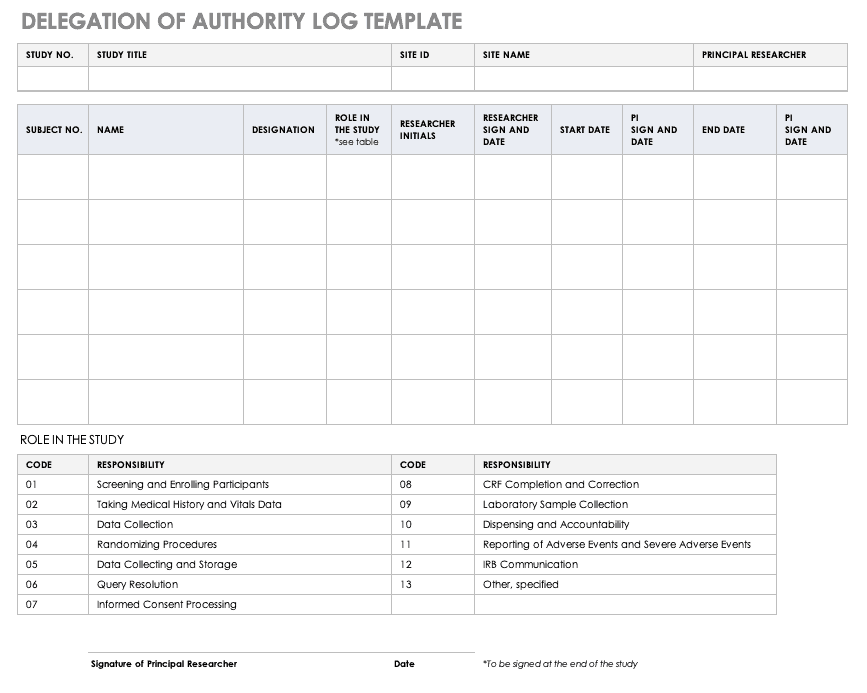
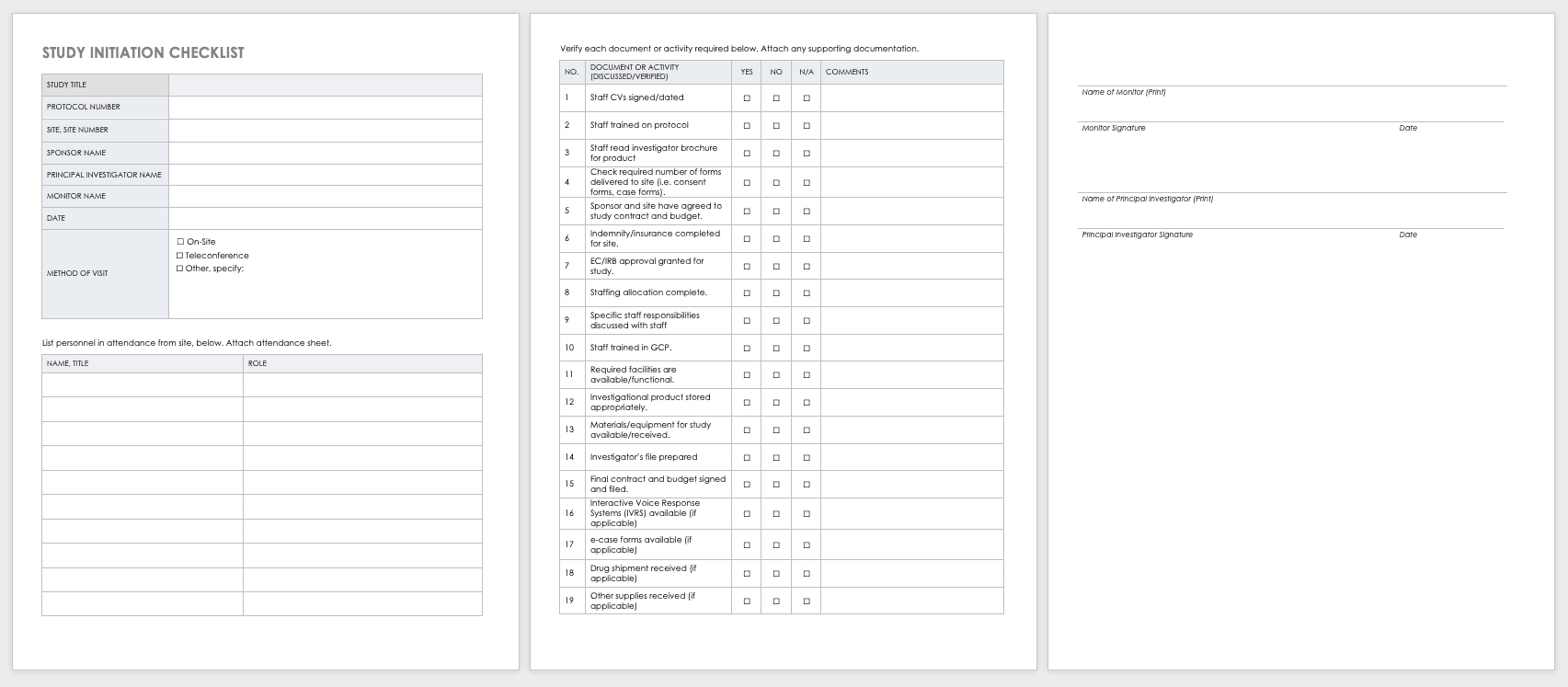
New Clinical Research Staff Orientation & Training Checklist
Free Clinical Trial Templates Smartsheet
Free Training Checklist Template Word Printable Templates
FREE Training Checklist Templates & Examples Edit Online & Download
Free Clinical Trial Templates Smartsheet
Though Not Legally Binding, Maintaining A.
Web A Resource Outlining The Steps Needed To Clear A Path Forward For Decentralized Clinical Trial Implementation
Please Consult The Main Clinical Research Toolkit Page For Information.
Selecting & Testing A Digital Health Technology.
Related Post: