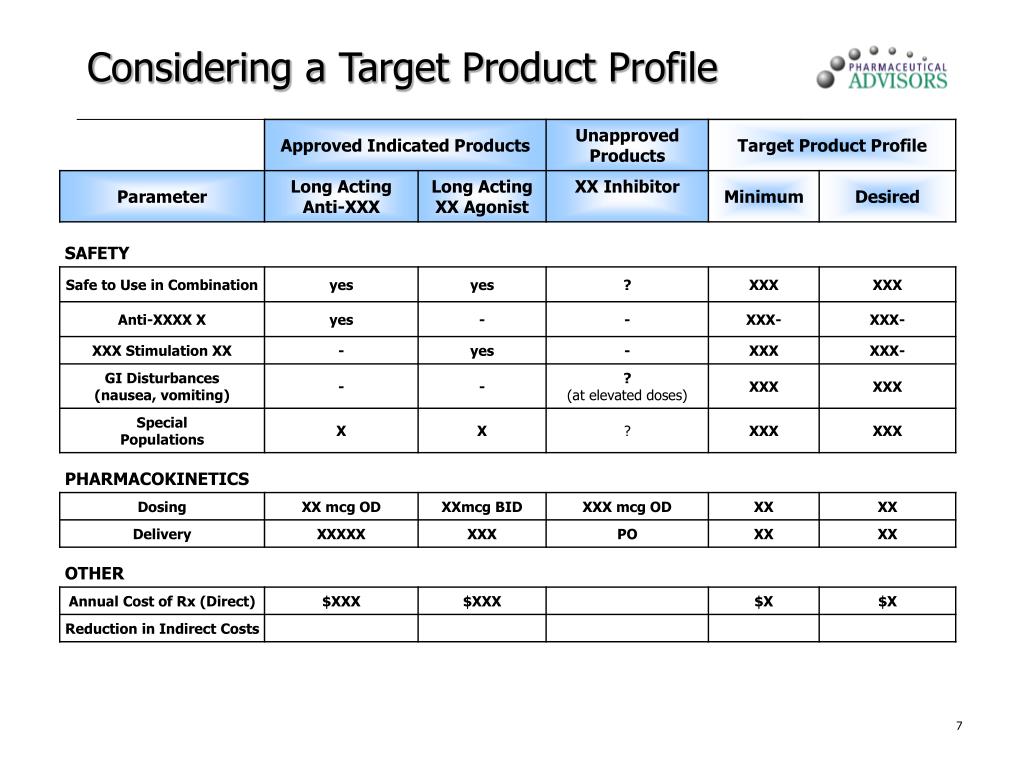
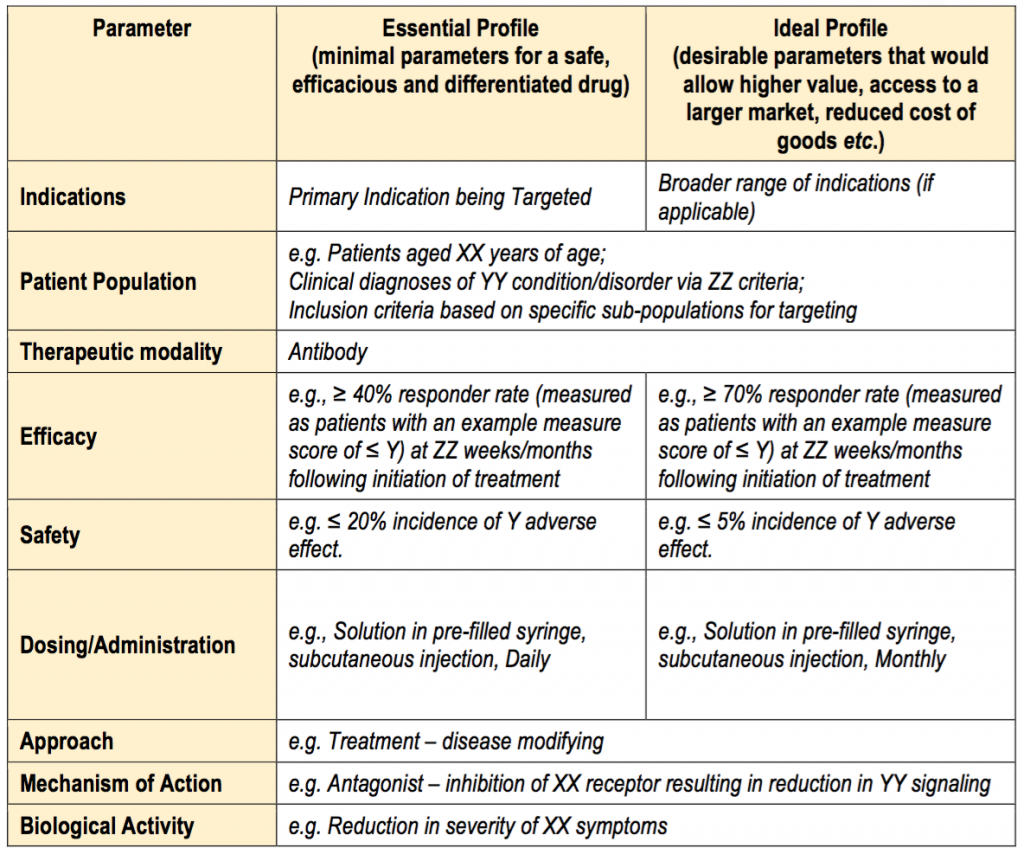
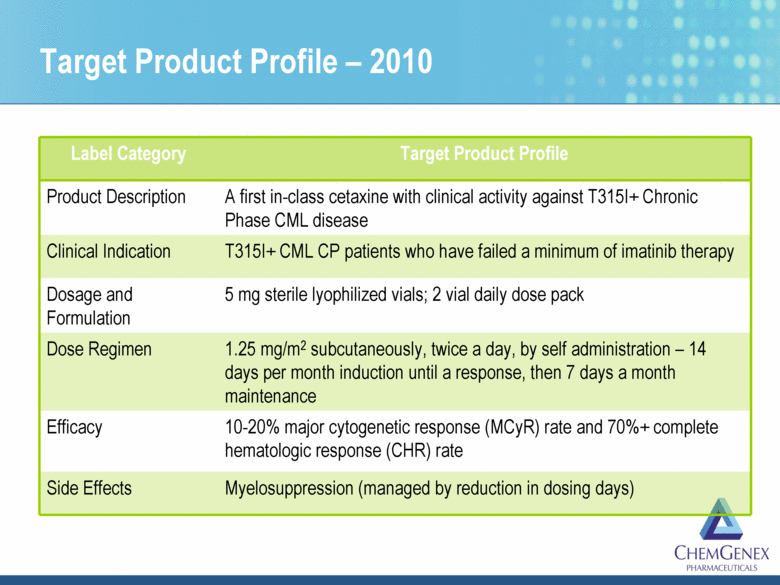
Target Product Profile Template
Target Product Profile Template - Web a target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidance for industry and review staff target product profile — a strategic development process tool. Web the generic product development plans (pdps) and target product profiles (tpps) below provide guidance to support product development activities through phase 1 clinical development: Web the drug development process. Web a target product profile (tpp) outlines the desired ‘profile’ or characteristics of a target product that is aimed at a particular disease or diseases. It includes both minimal and preferred product attributes and gives innovators a defined framework to evaluate their drug candidate as they move through the development process. Web below is an example worksheet based on the fda guidance that defines the minimal/ideal profile of the final marketed product and shows the ultimate goals of the proposed therapy development effort. Web target product profile template this template provides suggested considerations that may assist biopharmaceutical companies in their decisions as to whether to proceed with a drug development program (program). Web a target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidelines. The name given to this template was the target product profile. Below are example worksheets that define the minimal/ideal profile of the final marketed product and shows the ultimate goals of the proposed therapy. Below are example worksheets that define the minimal/ideal profile of the final marketed product and shows the ultimate goals of the proposed therapy. The name given to this template was the target product profile. Web a target product profile (tpp) outlines the desired ‘profile’ or characteristics of a target product that is aimed at a particular disease or diseases. The working group recommended use of a template that provides a summary of drug labeling concepts to focus discussions and aid in the understanding between sponsors and the fda. It includes both minimal and preferred product attributes and gives innovators a defined framework to evaluate their drug candidate as they move through the development process. Tpps state intended use, target populations and other desired attributes of products, including. Web the drug development process. Web a target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidance for industry and review staff target product profile — a strategic development process tool. The cber office of cellular, tissue and gene therapies (octgt) web page for industry education also has a webinar on tpp. Web below is an example worksheet based on the fda guidance that defines the minimal/ideal profile of the final marketed product and shows the ultimate goals of the proposed therapy development effort. Web the drug development process. Web target product profile template this template provides suggested considerations that may assist biopharmaceutical companies in their decisions as to whether to proceed with a drug development program (program). Web the generic product development plans (pdps) and target product profiles (tpps) below provide guidance to support product development activities through phase 1 clinical development: The cber office of cellular, tissue and gene therapies (octgt) web page for industry education also has a webinar on tpp. Web a target product profile (tpp) outlines the desired ‘profile’ or characteristics of a target product that is aimed at a particular disease or diseases. Web a target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidelines. The working group recommended use of a template that provides a summary of drug labeling concepts to focus discussions and aid in the understanding between sponsors and the fda. Tpps state intended use, target populations and other desired attributes of products, including. Below are example worksheets that define the minimal/ideal profile of the final marketed product and shows the ultimate goals of the proposed therapy. It includes both minimal and preferred product attributes and gives innovators a defined framework to evaluate their drug candidate as they move through the development process. Web target product profile template this template provides suggested considerations that may assist biopharmaceutical companies in their decisions as to whether to proceed with a drug development program (program). The working group recommended use of a template that provides a summary of drug labeling concepts to focus discussions and aid in the understanding between sponsors and the fda. Web below. It includes both minimal and preferred product attributes and gives innovators a defined framework to evaluate their drug candidate as they move through the development process. Tpps state intended use, target populations and other desired attributes of products, including. Web the drug development process. Web below is an example worksheet based on the fda guidance that defines the minimal/ideal profile. Web a target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidance for industry and review staff target product profile — a strategic development process tool. Web target product profile template this template provides suggested considerations that may assist biopharmaceutical companies in their decisions as to whether to proceed with a drug development program (program).. The working group recommended use of a template that provides a summary of drug labeling concepts to focus discussions and aid in the understanding between sponsors and the fda. The name given to this template was the target product profile. Web below is an example worksheet based on the fda guidance that defines the minimal/ideal profile of the final marketed. Web a target product profile (tpp) outlines the desired ‘profile’ or characteristics of a target product that is aimed at a particular disease or diseases. Web below is an example worksheet based on the fda guidance that defines the minimal/ideal profile of the final marketed product and shows the ultimate goals of the proposed therapy development effort. It includes both. Tpps state intended use, target populations and other desired attributes of products, including. Web below is an example worksheet based on the fda guidance that defines the minimal/ideal profile of the final marketed product and shows the ultimate goals of the proposed therapy development effort. The name given to this template was the target product profile. Web a target product. Web a target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidance for industry and review staff target product profile — a strategic development process tool. Web a target product profile (tpp) outlines the desired ‘profile’ or characteristics of a target product that is aimed at a particular disease or diseases. The name given to. Web target product profile template this template provides suggested considerations that may assist biopharmaceutical companies in their decisions as to whether to proceed with a drug development program (program). Web the drug development process. Web below is an example worksheet based on the fda guidance that defines the minimal/ideal profile of the final marketed product and shows the ultimate goals. The cber office of cellular, tissue and gene therapies (octgt) web page for industry education also has a webinar on tpp. It includes both minimal and preferred product attributes and gives innovators a defined framework to evaluate their drug candidate as they move through the development process. Web a target product profile (tpp) is a planning tool for therapeutic candidates. Web a target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidance for industry and review staff target product profile — a strategic development process tool. Web the generic product development plans (pdps) and target product profiles (tpps) below provide guidance to support product development activities through phase 1 clinical development: Web a target product. Web the generic product development plans (pdps) and target product profiles (tpps) below provide guidance to support product development activities through phase 1 clinical development: It includes both minimal and preferred product attributes and gives innovators a defined framework to evaluate their drug candidate as they move through the development process. Web a target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidance for industry and review staff target product profile — a strategic development process tool. Web below is an example worksheet based on the fda guidance that defines the minimal/ideal profile of the final marketed product and shows the ultimate goals of the proposed therapy development effort. The name given to this template was the target product profile. Below are example worksheets that define the minimal/ideal profile of the final marketed product and shows the ultimate goals of the proposed therapy. Web target product profile template this template provides suggested considerations that may assist biopharmaceutical companies in their decisions as to whether to proceed with a drug development program (program). Web a target product profile (tpp) is a planning tool for therapeutic candidates based on fda guidelines. Web a target product profile (tpp) outlines the desired ‘profile’ or characteristics of a target product that is aimed at a particular disease or diseases. Tpps state intended use, target populations and other desired attributes of products, including.Target Product Profile Template
Fda Target Product Profile Template
Target Product Profile Template
Fda Target Product Profile Template
Target Product Profile Fda Template
Target Product Profile Template
Target Product Profile Template
Product Profile Template
Target Product Profile Template
Target Product Profile Template
The Working Group Recommended Use Of A Template That Provides A Summary Of Drug Labeling Concepts To Focus Discussions And Aid In The Understanding Between Sponsors And The Fda.
The Cber Office Of Cellular, Tissue And Gene Therapies (Octgt) Web Page For Industry Education Also Has A Webinar On Tpp.
Web The Drug Development Process.
Related Post: