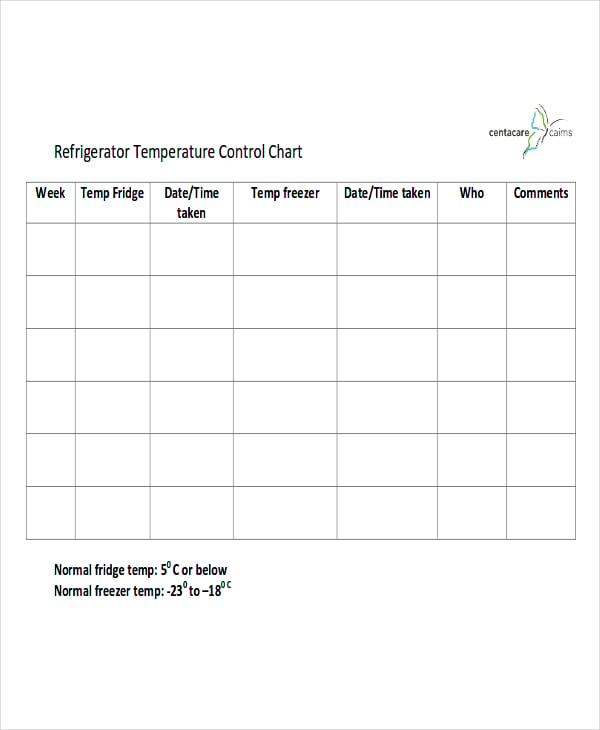
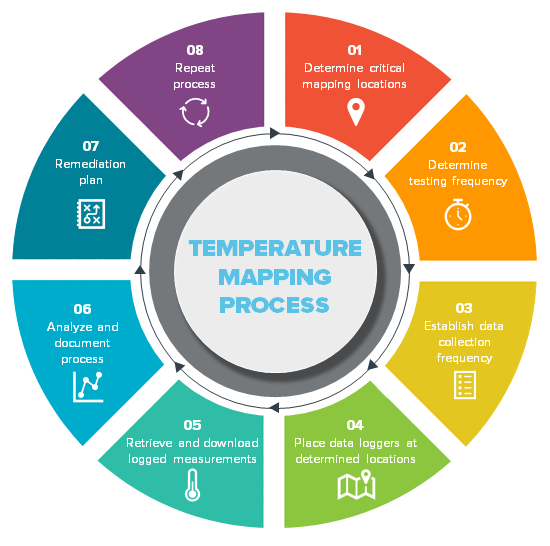
Temperature Mapping Protocol Template
Temperature Mapping Protocol Template - Web a properly developed qualification protocol will help ensure that your equipment meets all documentation requirements and performs as expected, including the temperature mapping study is. Web • provides guidance on good practices for the mapping of controlled temperature chambers, warehouses, and refrigerated storage areas used to store raw material, work in progress, or finished product and which operate under current gmp. Historically, mapping of cleanrooms has not been performed beyond a single point temperature or humidity verification during qualification because. Web temperature mapping study analyzes the distribution of temperature and humidity. Store below 25°c/store below 30°c. How often should you do mappings? To cover the full range of temperature regimes, a standard protocol can be used to map any storage area in the facility. Web in principle a temperature mapping study involves recording the temperature of the cold compartment under various conditions. So, why should we consider mapping cleanrooms? Verify that the refrigerator is properly installed according to the manufacturer and xxxxxxx Document and schedule mapping study. What do we do with the data? When should you do it? To lay down a procedure for conduct a temperature mapping exercise in store. Web temperature mapping study to analyze distribution of temperature inside the whole area. What testing should you do? Who to include in your temperature mapping. The purpose of the temperature mapping protocol is to ensure the mapping exercise can be carried out in the correct manner. The instructions are compatible with the use of the following temperature data logger: The equipment you need for mapping. We have to collect the data Web in principle a temperature mapping study involves recording the temperature of the cold compartment under various conditions. Specficis for different types of units. The purpose of the temperature mapping protocol is to ensure the mapping exercise can be carried out in the correct manner. Store where raw materials, packing material and finished products are stored under specified temperature conditions. So, why should we consider mapping cleanrooms? It should be prepared, reviewed and approved by someone who has. Store below 25°c/store below 30°c. Key guidelines and regulations for. What do we do with the data? When should you do it? Web temperature mapping study to analyze distribution of temperature inside the whole area. The data will be recorded for days or weeks as per Document and schedule mapping study. Web on this page, you can delve into: Web this is a user guide for the temperature mapping tool version 7 published on 22 november 2021. Web this document discusses guidelines for temperature mapping studies and qualification of cold storage facilities, vehicles, and packaging used in the pharmaceutical industry. Briefly describe the equipment, its major components and their roles. Web temperature mapping study analyzes the distribution of temperature. Web • provides guidance on good practices for the mapping of controlled temperature chambers, warehouses, and refrigerated storage areas used to store raw material, work in progress, or finished product and which operate under current gmp. To cover the full range of temperature regimes, a standard protocol can be used to map any storage area in the facility. Web this. What do we do with the data? Web standard operating procedure of temperature mapping study in biological incubators, refrigerator and freezer using calibrated probes and data logger in pharmaceutical industries. We have to collect the data The data will be recorded for days or weeks as per The mapping protocol should contain the following sections: Historically, mapping of cleanrooms has not been performed beyond a single point temperature or humidity verification during qualification because. Web in this article, we’ll cover the basics of temperature mapping in pharma, how it’s done, and some of the key elements to include in a temperature mapping protocol and validation. Web in principle, a temperature mapping study involves recording the. The mapping protocol should contain the following sections: Document and schedule mapping study. Briefly describe the equipment, its major components and their roles. The equipment you need for mapping. This sop shall be applicable to cold storage, foil store, bsr and r.m. What do we do with the data? 5 steps to perform temperature mapping in gxp. We carry out these tests to check the uniformity of the temperature within the area. The equipment you need for mapping. This sop shall be applicable to cold storage, foil store, bsr and r.m. It should be prepared, reviewed and approved by someone who has. The equipment you need for mapping. Web the document outlines a temperature mapping protocol to identify temperature variations within vaccine storage areas. What testing should you do? To cover the full range of temperature regimes, a standard protocol can be used to map any storage area in the facility. So, why should we consider mapping cleanrooms? Web in principle, a temperature mapping study involves recording the temperature of the cold room, warehouse or any such asset under various conditions. Do not store above 25°c/do not store above 30°c. Web temperature mapping protocol. Web temperature mapping study to analyze distribution of temperature inside the whole area. Conduct test and review data. 5 steps to perform temperature mapping in gxp. Web in principle a temperature mapping study involves recording the temperature of the cold compartment under various conditions. Web where is it required? Web this is a user guide for the temperature mapping tool version 7 published on 22 november 2021. The equipment you need for mapping. Document and schedule mapping study. Examples of specific storage statements declared on the label of a medicinal product: Store where raw materials, packing material and finished products are stored under specified temperature conditions. Web this document discusses guidelines for temperature mapping studies and qualification of cold storage facilities, vehicles, and packaging used in the pharmaceutical industry. Conduct test and review data. This sop shall be applicable to cold storage, foil store, bsr and r.m. Web this is a user guide for the temperature mapping tool version 7 published on 22 november 2021. Historically, mapping of cleanrooms has not been performed beyond a single point temperature or humidity verification during qualification because. By conducting thorough temperature mapping studies, organizations can identify risk areas, mitigate temperature fluctuations, and take proactive measures to maintain product quality throughout the. Web • provides guidance on good practices for the mapping of controlled temperature chambers, warehouses, and refrigerated storage areas used to store raw material, work in progress, or finished product and which operate under current gmp. The instructions are compatible with the use of the following temperature data logger: We carry out these tests to check the uniformity of the temperature within the area. Web on this page, you can delve into: It should be prepared, reviewed and approved by someone who has. Specficis for different types of units.Temperature Mapping Protocol Template
Temperature Mapping Protocol Template
Temperature Mapping Protocol Template
Temperature Mapping Protocol Template
Temperature Mapping Protocol Template
Temperature Mapping Protocol Template
Temperature Mapping Protocol Template
Temperature Mapping Protocol Template
The complete industrial guide to temperature mapping
Temperature Mapping Protocol Template
When Should You Do It?
Web Temperature Mapping Study Analyzes The Distribution Of Temperature And Humidity.
To Lay Down A Procedure For Conduct A Temperature Mapping Exercise In Store.
Web Where Is It Required?
Related Post: