Toxicology Report Template
Toxicology Report Template - These reports serve as valuable resources for medical professionals, law enforcement agencies, and regulatory authorities. Adverse effects of xenobiotics on the health of humans. The journal strives to provide a forum for original research papers pertaining to, but not limited to, the following subjects: Web toxicology reports template will format your research paper to elsevier's guidelines. How to evaluate data and write reports e dited by g. Web toxicology reporting specific solutions for toxicology labs. Web physiologically based pharmacokinetic (pbpk) model reporting template for chemical risk assessment applications. Web templates for reporting toxicology data in support of petitions and notifications for food and color additives. Web how to evaluate data and write reports. N oh yn ek wa sh in gton : Volatiles (e.g., chloroform, ethanol [alcohol], acetone, isopropanol, methanol and toluene) illicit drugs (e.g., heroin, cocaine, marijuana, pcp, methamphetamine) These reports serve as valuable resources for medical professionals, law enforcement agencies, and regulatory authorities. Web a toxicological risk assessment (tra) is a safety evaluation of a product based on its composition and intended uses. View full aims & scope. Web our toxicology results are easy to read and provide essential information to make informed decisions on drug detection and monitoring. And indicate contents, which are required to evaluate the toxicity of a tested compound. Web how to evaluate data and write reports. Web typical drugs and substances that may undergo toxicology screening for a forensic toxicology report include: Web nonclinical common report template. Note that these are sample reports generated with tools available in orchard software’s laboratory solutions. Web peer review the following chapters within the draft specifications for the conduct of toxicity studies by the division of translational toxicology at the national institute of environmental health sciences. Web how to evaluate data and write reports. Web our toxicology results are easy to read and provide essential information to make informed decisions on drug detection and monitoring. Web sample reports generated from orchard software test database. A laboratory information management system (lims) is crucial in overcoming challenges linked with toxicology reporting, boosting efficiency, precision, and overall workflow in diagnostic labs. Summarize essential knowledge and key changes regarding exposure, poisons, and poisoning; Volatiles (e.g., chloroform, ethanol [alcohol], acetone, isopropanol, methanol and toluene) illicit drugs (e.g., heroin, cocaine, marijuana, pcp, methamphetamine) Environmental protection agency office of pesticide programs november 11, 2021. Web signs associated with fentanyl toxicity include severe respiratory depression, seizures, hypotension, coma and death. Web templates for reporting toxicology data in support of petitions and notifications for food and color additives. Note that these are sample reports generated with tools available in orchard software’s laboratory solutions. Web templates for reporting toxicology data in support of petitions and notifications for food and color additives. Web toxicology reports is dedicated to all aspects of toxicology research and clinical sciences. Web a toxicological risk assessment (tra) is a safety evaluation of a product based. Ta ylor & fran cis, 139 pp., $29.95 reviewed by arth u r f u rst, u n iversity of san f ra n cisco, san fra n cisco, california th is is a differen t book. View full aims & scope. Specific report formatting assistance from orchard software may require a professional services agreement. Web toxicology reporting specific solutions. Summarize essential knowledge and key changes regarding exposure, poisons, and poisoning; Web to provide guidance and a standard procedure for conducting data audits of acute, subchronic, and chronic general toxicology studies intended to be submitted to the environmental protection agency (epa) to assure compliance with the glp standards (40 cfr part 160 [fifra] and 40 cfr part 792 [tsca]. Adverse. Society of toxicology, biological modeling specialty section webiniar. Web to provide guidance and a standard procedure for conducting data audits of acute, subchronic, and chronic general toxicology studies intended to be submitted to the environmental protection agency (epa) to assure compliance with the glp standards (40 cfr part 160 [fifra] and 40 cfr part 792 [tsca]. Web sample reports generated. Developed as a companion to the nonclinical common protocol template, the report template is also now available for voluntary adoption. Volatiles (e.g., chloroform, ethanol [alcohol], acetone, isopropanol, methanol and toluene) illicit drugs (e.g., heroin, cocaine, marijuana, pcp, methamphetamine) In fatalities from fentanyl, blood concentrations are variable and have been reported as low as 3 ng/ml. Web sample reports generated from. Note that these are sample reports generated with tools available in orchard software’s laboratory solutions. Volatiles (e.g., chloroform, ethanol [alcohol], acetone, isopropanol, methanol and toluene) illicit drugs (e.g., heroin, cocaine, marijuana, pcp, methamphetamine) Download your paper in word & latex, export citation & endnote styles, find journal impact factors, acceptance rates, and more. Environmental protection agency office of pesticide programs. Web to provide guidance and a standard procedure for conducting data audits of acute, subchronic, and chronic general toxicology studies intended to be submitted to the environmental protection agency (epa) to assure compliance with the glp standards (40 cfr part 160 [fifra] and 40 cfr part 792 [tsca]. View full aims & scope. And indicate contents, which are required to. Web a toxicological risk assessment (tra) is a safety evaluation of a product based on its composition and intended uses. Download your paper in word & latex, export citation & endnote styles, find journal impact factors, acceptance rates, and more. View full aims & scope. Web peer review the following chapters within the draft specifications for the conduct of toxicity. N oh yn ek wa sh in gton : Volatiles (e.g., chloroform, ethanol [alcohol], acetone, isopropanol, methanol and toluene) illicit drugs (e.g., heroin, cocaine, marijuana, pcp, methamphetamine) Written in such a way as to make it accessible to toxicologists who do not have english as a first language, this book focuses on evaluating, interpreting and reporting results of regulatory toxicology. Web written in such a way as to make it accessible to toxicologists who do not have english as a first language, this book focuses on evaluating, interpreting and reporting results of regulatory toxicology studies. Web typical drugs and substances that may undergo toxicology screening for a forensic toxicology report include: Web this guideline describes an acceptable format for organizing. The journal strives to provide a forum for original research papers pertaining to, but not limited to, the following subjects: Web how to evaluate data and write reports. Environmental protection agency office of pesticide programs november 11, 2021. How to evaluate data and write reports e dited by g. Ta ylor & fran cis, 139 pp., $29.95 reviewed by arth u r f u rst, u n iversity of san f ra n cisco, san fra n cisco, california th is is a differen t book. Web toxicology reports are essential documents that present vital information about the presence of toxins and their impact on individuals and the environment. Summarize essential knowledge and key changes regarding exposure, poisons, and poisoning; Web nonclinical common report template. Web toxicology reports template will format your research paper to elsevier's guidelines. Web what is a toxicology report/statement? Web sample reports generated from orchard software test database. Web peer review the following chapters within the draft specifications for the conduct of toxicity studies by the division of translational toxicology at the national institute of environmental health sciences. Web toxicology reporting specific solutions for toxicology labs. Developed as a companion to the nonclinical common protocol template, the report template is also now available for voluntary adoption. To be specific, a toxicology report, which is also referred to as the expert statement, is a document detailing the detection, isolation, and identification of chemicals, toxins, or poisons in a given sample. Written in such a way as to make it accessible to toxicologists who do not have english as a first language, this book focuses on evaluating, interpreting and reporting results of regulatory toxicology studies.Toxicology Report Template Blank Autopsy Report Form Success in Coroner
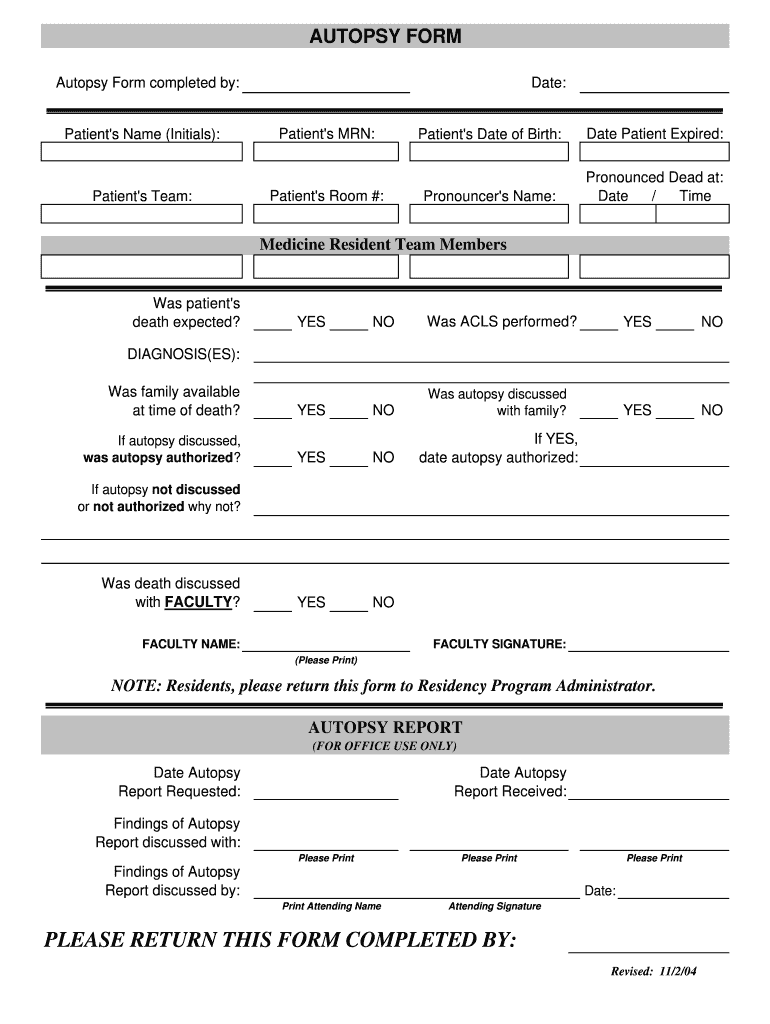
Autopsy Report Template Fill Online, Printable, Fillable, Blank
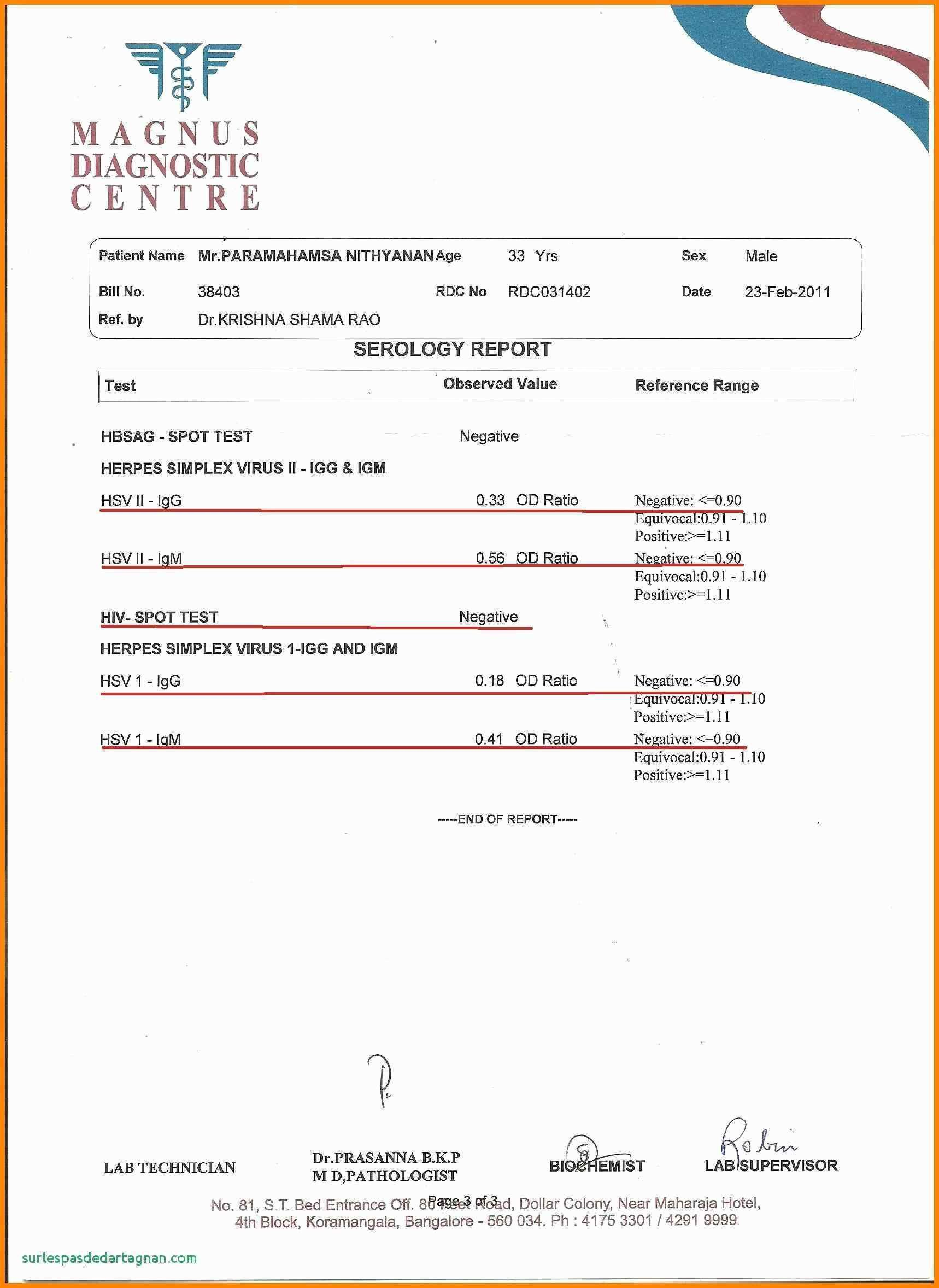
Toxicology Report Sample intended for Fascinating Blank Autopsy Report
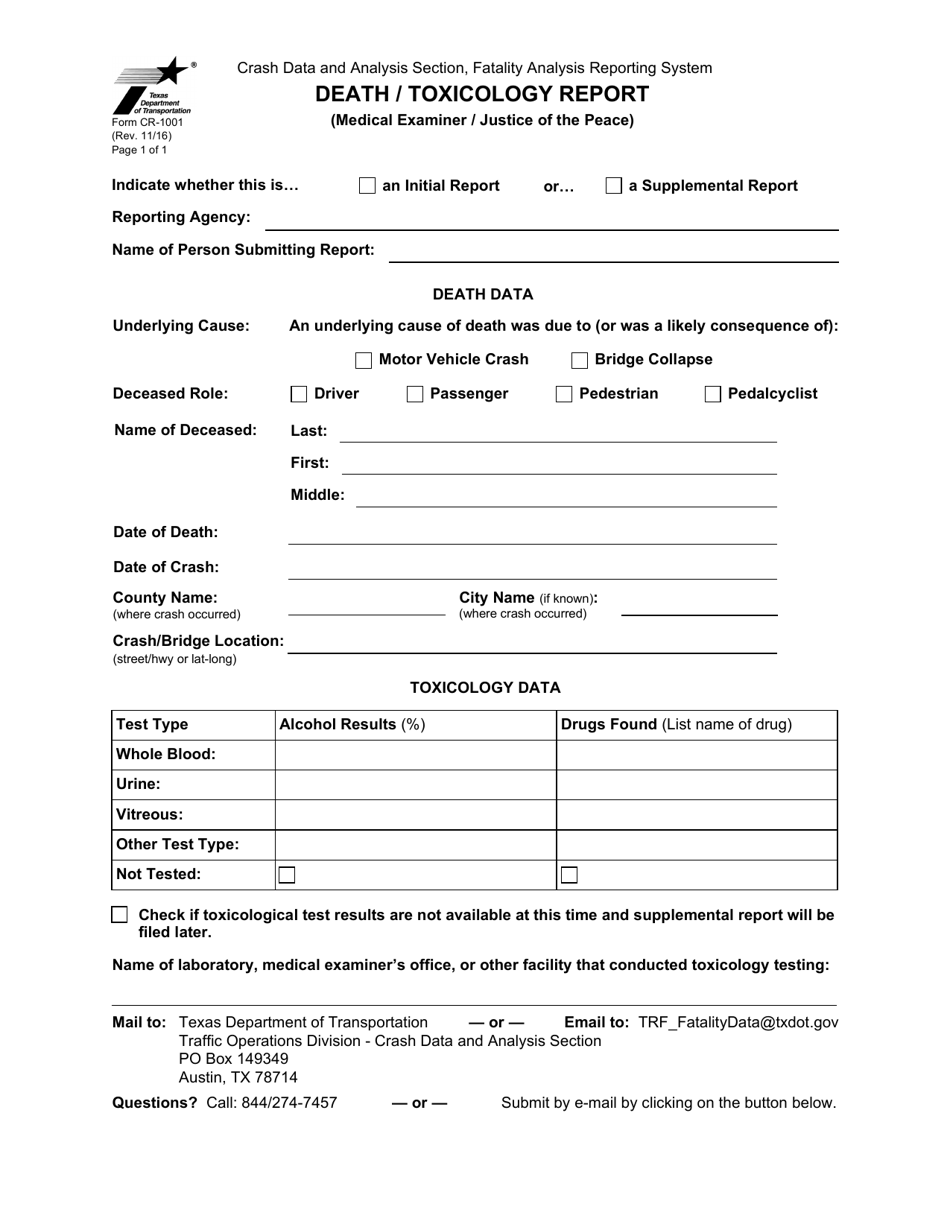
Toxicology Report Template
Toxicology Report Template
Pinterest
Toxicology Report Template
Toxicology Report Template
Toxicology Report Template
Toxicology Report Template
And Indicate Contents, Which Are Required To Evaluate The Toxicity Of A Tested Compound.
Adverse Effects Of Xenobiotics On The Health Of Humans.
Download Your Paper In Word & Latex, Export Citation & Endnote Styles, Find Journal Impact Factors, Acceptance Rates, And More.
Volatiles (E.g., Chloroform, Ethanol [Alcohol], Acetone, Isopropanol, Methanol And Toluene) Illicit Drugs (E.g., Heroin, Cocaine, Marijuana, Pcp, Methamphetamine)
Related Post: