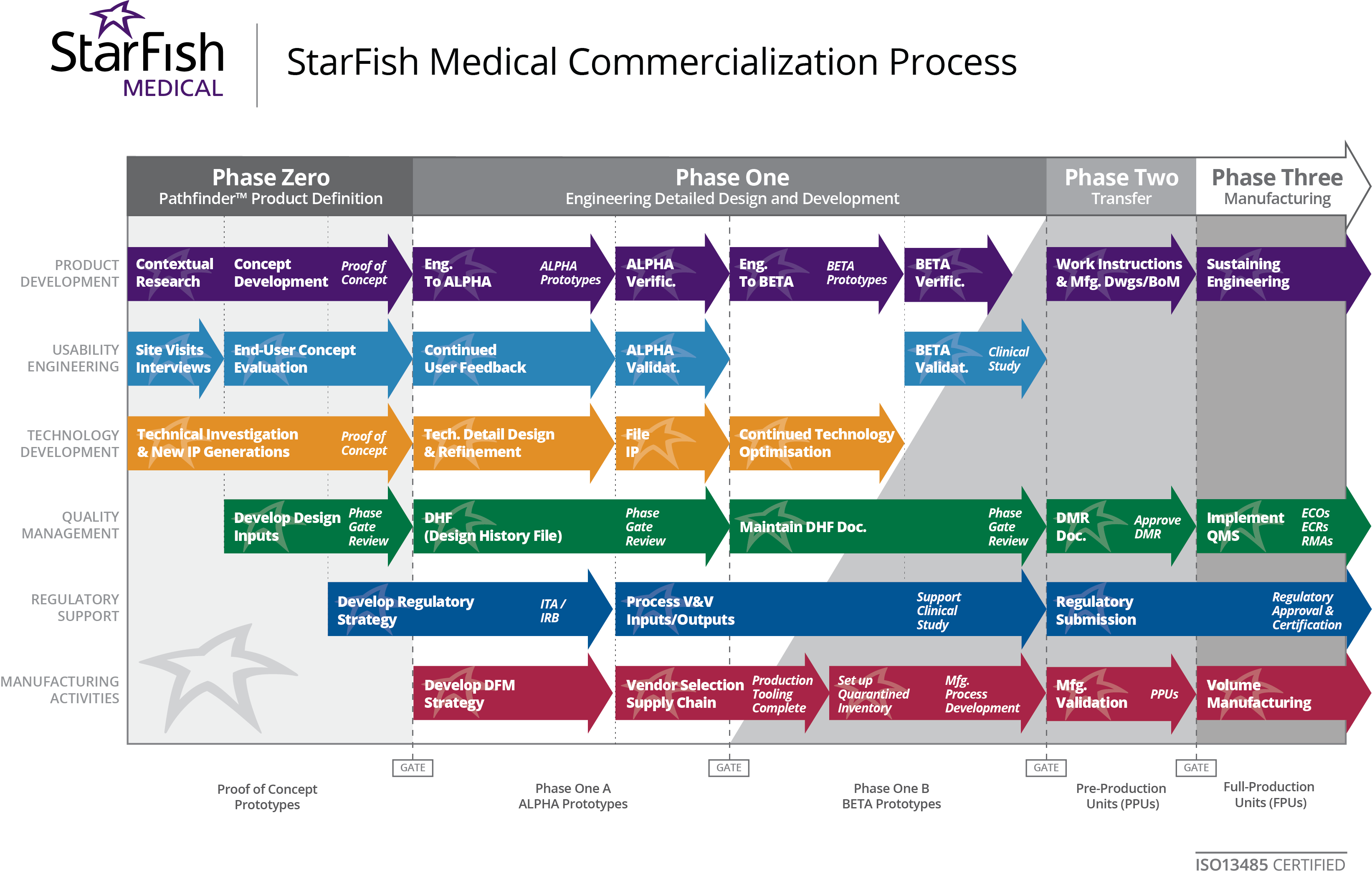
Sample Of Medical Device Quality Plan Template
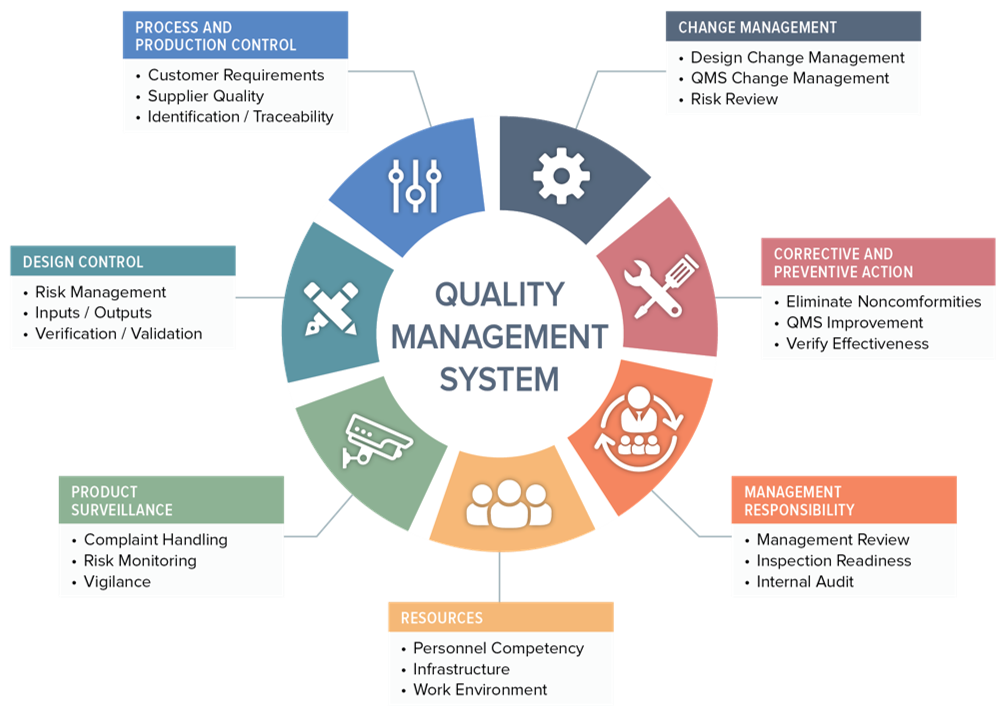
Sample Of Medical Device Quality Plan Template - Web in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms) in the medical device industry, which begs the questions: Web quality manual template for medical device manufacturers, according to iso 13485 and regulation (eu) 2017/745. Identify inputs for the quality plan. The iso 13485 is the standard for quality management in the medical device industry. Web this article explains how to write a quality system plan template to revise and update your quality system for compliance with iso 13485:2016. Monitor and evaluate the plan’s outputs. This powerpoint template showcases a detailed marketing plan for pharmacies and medical devices. Use these 8 templates to get started. Additionally, you must define how the project will be controlled and how deliverables will be managed. Pharmacy and medical devices marketing strategies and plan. Web a look at our eu mdr templates for medical devices. If you want to download our free template, there is a form to complete at the end of this article. Pharmacy and medical devices marketing strategies and plan. What is a medical device quality management system (qms)? Cockpit provides a template for design controls that help you meet the fda’s best practice standards outlined in 21 cfr 820.30. Regulatory requirements for medical device companies are stricter than ever before. Web medical device quality policy examples. Web quality manual template for medical device manufacturers, according to iso 13485 and regulation (eu) 2017/745. What are quality objectives, what is their purpose, and. Web this guide will: Provide a useful overview of considerations when implementing and maintaining a qms, address common pitfalls and how to avoid them, and. The iso 13485 is the standard for quality management in the medical device industry. What are quality objectives, what is their purpose, and. Additionally, you must define how the project will be controlled and how deliverables will be managed. In this step, you will identify the inputs required for the quality plan of your medical device. Download them for free and get your compliance done, no strings attached. Determine the scope of the quality plan. Web the site quality plan should describe how your company’s quality policy allows creation of quality procedures (maintained in the quality manual) that satisfy local and federal regulations as well as quality system practices (i.e., the qsr, iso 13485). Pharmacy and medical devices marketing strategies and plan. Additionally, we publish all our document templates for the iso 13485 for free, so scroll. Additionally, you must define how the project will be controlled and how deliverables will be managed. Regulatory requirements for medical device companies are stricter than ever before. Here are all our posts on this standard, and also all questions our consulting clients have asked us about the iso 13485 so far. Our templates currently cover compliance for iso 13485, iec. Not all medical devices are required to adhere to the design control standards, so make sure to read through 21 cfr 820.30 to find out if you have to comply. Use these 8 templates to get started. Regulatory requirements for medical device companies are stricter than ever before. The cer is where the meat of your presentation, analysis and discussion. In this step, you will identify the inputs required for the quality plan of your medical device. Pharmacy and medical devices marketing strategies and plan. Additionally, we publish all our document templates for the iso 13485 for free, so scroll. This manual covers the quality system regulation and the basic good manufacturing practices (gmp) requirements that all manufacturers and distributors. Identify inputs for the quality plan. Web this guide will: The documentation template may be used for iso 13485 certification audit purposes. Web a look at our eu mdr templates for medical devices. Web learn what the iso 13485 quality policy is and why your qms needs it. Web in clause 4.2.1, iso 13485 lists quality objectives as being mandatory for a quality management system (qms) in the medical device industry, which begs the questions: This manual provides comprehensive evidence to all customers, suppliers and employees of what specific controls are implemented to ensure. The iso 13485 is the standard for quality management in the medical device industry.. The iso 13485 is the standard for quality management in the medical device industry. Web this article explains how to write a quality system plan template to revise and update your quality system for compliance with iso 13485:2016. Our templates currently cover compliance for iso 13485, iec 62304, iso 14971 and iec 62366. Web the qms is integral in ensuring. Web medical device quality policy examples. Use these 8 templates to get started. Provide a useful overview of considerations when implementing and maintaining a qms, address common pitfalls and how to avoid them, and. More health centres, more countries, increase in diversity of age / race ) Monitor and evaluate the plan’s outputs. In this step, you will identify the inputs required for the quality plan of your medical device. The clinical evaluation report (cer) template. Web this article explains how to write a quality system plan template to revise and update your quality system for compliance with iso 13485:2016. More health centres, more countries, increase in diversity of age / race ). Determine the scope of the quality plan. This powerpoint template showcases a detailed marketing plan for pharmacies and medical devices. If you want to download our free template, there is a form to complete at the end of this article. A detailed guide to quality management systems. Web steps to create a medical device quality plan. The clinical evaluation report (cer) template. Web a medical device quality plan is not only required by the fda and the iso 13485 framework, but it can help you build quality into your product and company from day one. Identify inputs for the quality plan. Web quality manual template for medical device manufacturers, according to iso 13485 and regulation (eu) 2017/745. Pharmacy and medical devices marketing strategies and plan. Web this plan should outline your goals, objectives, and strategies for maintaining the quality of your devices throughout the development process. What is a medical device quality management system (qms)? Web this article explains how to write a quality system plan template to revise and update your quality system for compliance with iso 13485:2016. Additionally, we publish all our document templates for the iso 13485 for free, so scroll. Web learn what the iso 13485 quality policy is and why your qms needs it. Explain what a quality management system (qms) is. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. A detailed guide to quality management systems. In this step, you will identify the inputs required for the quality plan of your medical device. Web the qms is integral in ensuring that the development, manufacturing and other operational processes fulfil quality and safety requirements, thereby producing medical devices that are safe, effective and regulatory compliant. Our templates currently cover compliance for iso 13485, iec 62304, iso 14971 and iec 62366.Medical Device QMS 101 What It Is, Where It’s Required, and Key
Medical Device Quality Plan Template
Medical Device Product Development Plan Template Google Docs, Word
Medical Device Quality Plan Template
Free Free Sample Quality Management Plan Template Google Docs, Word
Medical Device Project Plan Template
Medical Device Design And Development Plan Template
Medical Device Quality Agreement Template in PDF, Word, Google Docs
Medical Device Quality Plan Template
The Iso 13485 Is The Standard For Quality Management In The Medical Device Industry.
If You Want To Download Our Free Template, There Is A Form To Complete At The End Of This Article.
This Includes Listing, Reviewing, Verifying, Approving, And Distributing Documents.
Web This Guide Will:
Related Post: